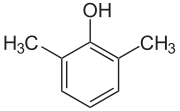
2,6-Xylenol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,6-Dimethylphenol | |
| Other names
2-Hydroxy-m-xylene | |
| Identifiers | |
| 576-26-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 13839174 |
| ECHA InfoCard | 100.008.547 |
| PubChem | 11335 |
| UNII | I8N0RO87OV |
| |
| |
| Properties | |
| C8H10O | |
| Molar mass | 122.17 g·mol−1 |
| Melting point | 43 to 45 °C (109 to 113 °F; 316 to 318 K) |
| Boiling point | 203 °C (397 °F; 476 K) |
| Hazards | |
| Flash point | 86 °C (187 °F; 359 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
2,6-Xylenol is a chemical compound which is one of the six isomers of xylenol. It is also commonly known as 2,6-dimethylphenol (DMP).
Related polymers
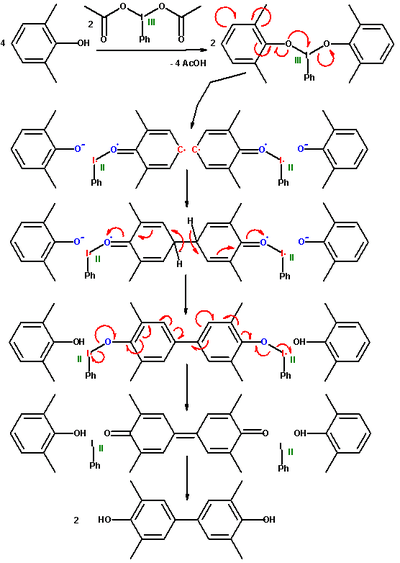
2,6-Xylenol is a monomer for poly(p-phenylene oxide) engineering resins through carbon - oxygen oxidative coupling. Carbon-to-carbon dimerization is also possible. In one study 2,6-xylenol is oxidized with iodosobenzene diacetate with a fivefold excess of the phenol.[2]
In the first step of the proposed reaction mechanism the acetyl groups in the iodine compound are replaced with the phenol. This complex dissociates into an aryl radical anion and a phenoxy residue. The two aryl radicals recombine forming a new carbon carbon covalent bond and subsequently lose two protons in a rearomatization step. The immediate reaction product is a diphenoquinone as result of a one-step 4-electron oxidation. It is nevertheless possible to synthesize the biphenol compound via a comproportionation of the quinone with xylenol already present. In this reaction sequence the hypervalent iodine reagent is eventually reduced to phenyliodine.
References
- ↑ 2,6-Xylenol at Sigma-Aldrich
- ↑ Selective oxidative para C–C dimerization of 2,6-dimethylphenol Christophe Boldron, Guillem Aromí, Ger Challa, Patrick Gamez and Jan Reedijk Chemical Communications, 2005, (46), 5808 - 5810 Abstract