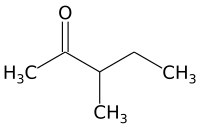
3-Methyl-2-pentanone
 | |
| Names | |
|---|---|
| IUPAC name
3-Methyl-2-pentanone | |
| Other names
3-Methylpentan-2-one; Methyl sec-butyl ketone | |
| Identifiers | |
| 565-61-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 10788 |
| ECHA InfoCard | 100.008.439 |
| EC Number | 209-282-1 |
| PubChem | 11262 |
| |
| |
| Properties | |
| C6H12O | |
| Molar mass | 100.16 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Peppermint-like |
| Density | 0.8130 g/mL (20 °C) |
| Melting point | −83 °C (−117 °F; 190 K) |
| Boiling point | 116 °C (241 °F; 389 K) |
| 2.26 wt % (20 °C) | |
| Refractive index (nD) |
1.4012 (20 °C) |
| Hazards | |
| EU classification (DSD) |
|
| Flash point | 12 °C (54 °F) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
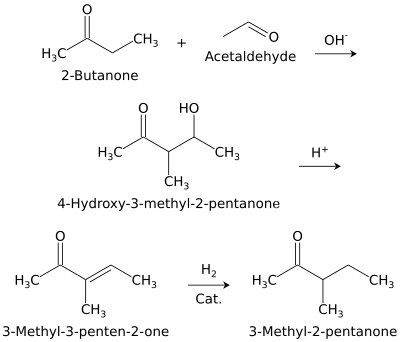
3-Methyl-2-pentanone (methyl sec-butyl ketone) is an aliphatic ketone and isomer of 2-hexanone. It is used as a solvent and as an intermediate for syntheses. Its industrial importance is low. It is produced by base-catalyzed aldol condensation of 2-butanone with acetaldehyde, forming 4-hydroxy-3-methyl-2-pentanone, which is dehydrated to 3-methyl-3-penten-2-one over an acid catalyst, followed by hydrogenation over a palladium catalyst.[1]
References
- ↑ Hardo Siegel, Manfred Eggersdorfer (2007), "Ketones", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 5
This article is issued from Wikipedia - version of the 11/2/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.