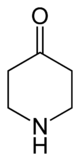
4-Piperidinone
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Piperidin-4-one | |
| Other names
4-Piperidone Azinanone Azinan-4-one | |
| Identifiers | |
| 41661-47-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 31091 |
| ECHA InfoCard | 100.050.420 |
| |
| |
| Properties | |
| C5H9NO | |
| Molar mass | 99.13 g·mol−1 |
| Boiling point | 79 °C (174 °F; 352 K) |
| Hazards | |
| EU classification (DSD) |
Flammable (F) Harmful (Xn) Dangerous for the environment (N) |
| NFPA 704 | |
| Flash point | 91 °C (196 °F; 364 K) |
| Related compounds | |
| Related compounds |
Piperidine; 2-Piperidinone |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
4-Piperidinone is a derivative of piperidine with the molecular formula C5H9NO. 4-Piperidone is used as an intermediate in the manufacture of chemicals and pharmaceutical drugs (e.g., fentanyl).
Piperidones
Piperidones are a class of chemical compounds sharing the piperidone skeleton. A classic named reaction for the synthesis of piperidones is the Petrenko-Kritschenko piperidone synthesis which involves combining an alkyl-1,3-acetonedicarboxylate with benzaldehyde and an amine.[1] This multicomponent reaction is related to the Hantzsch pyridine synthesis.
1-Methyl-4-piperidone
- used to make dorastine, Propiverine. 1,3-dimethyl-4-piperidone is used to make naranol, etc.
1-Benzyl-4-piperidone
- used to make: carpipramine, clocapramine, Fluspirilene, pipamperone, Benzetimide, Aplaviroc, & Osanetant.
N-Benzyl-4-piperidone is made when 1 equivalent of benzylamine is condensed with 2 molecules of ethylacrylate and the double conjugate addition product is subject of a Dieckmann cyclization followed by saponification and decarboxylation. Note that the intermediate 3-carboethoxy-N-Benzyl-4-piperidone prior to saponification can be used as a product to make Pimozide, Benperidol and droperidol.
N-Carboethoxy-4-piperidone was used to make Lorcainide.
References
- ↑ Ueber die Condensation von Aceton-dicarbonsäureestern mit Benzaldehyd unter Anwendung von Ammoniak P. Petrenko-Kritschenko, N. Zoneff Berichte der deutschen chemischen Gesellschaft Volume 39 Issue 2, Pages 1358 - 1361 1906 doi:10.1002/cber.19060390234
