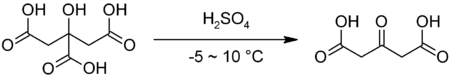Acetonedicarboxylic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-Oxopentanedioic acid | |
Other names
| |
| Identifiers | |
| 542-05-2 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChemSpider | 61623 |
| ECHA InfoCard | 100.007.999 |
| EC Number | 208-797-9 |
| PubChem | 68328 |
| |
| |
| Properties | |
| C5H6O5 | |
| Molar mass | 146.09814 g/mol |
| Density | 1.499 g/cm3 |
| Melting point | 122 °C (252 °F; 395 K) (decomposes) |
| Boiling point | 408.4 °C (767.1 °F; 681.5 K) (760mm Hg) |
| Hazards | |
| Flash point | 214.9 °C (418.8 °F; 488.0 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Acetonedicarboxylic acid, 3-oxoglutaric acid or β-ketoglutaric acid is a simple dicarboxylic acid.
Preparation
It is commercially available but may also be prepared by decarbonylation of citric acid in fuming sulfuric acid:[2]
Applications
Acetonedicarboxylic acid and its derivatives are primarily used as building blocks in organic chemistry, particularly in the synthesis of heterocyclic rings[3] and in the Weiss–Cook reaction.
Acetonedicarboxylic acid is well-known to be used in the Robinson tropinone synthesis.
Acetonedicarboxylic acid also finds use in the synthesis of Timcodar & dezaguanine (3-deazaguanine, 3-DG).
The presence of β-ketoglutaric acid in human urine can be used as a diagnostic test for the overgrowth of harmful gut flora such as Candida albicans.[4]
See also
References
- ↑ 1,3-Acetonedicarboxylic acid at Sigma-Aldrich (safety data sheet)
- ↑ Roger Adams; H. M. Chiles; C. F. Rassweiler (1941). "Acetonedicarboxylic Acid". Org. Synth.; Coll. Vol., 1, p. 10
- ↑ Stanovnik, Branko; Grošelj, Uroš (2010). "CHAPTER 5 – Dialkyl Acetone-1,3-Dicarboxylates and their Mono- and bis(Dimethylamino)methylidene Derivatives in the Synthesis of Heterocyclic Systems". Advances in Heterocyclic Chemistry. 100: 145–174. doi:10.1016/S0065-2725(10)10005-1.
- ↑ Schmidt, Michael A, Tired of Being Tired: Overcoming Chronic Fatigue and Low Energy
This article is issued from Wikipedia - version of the 11/27/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.