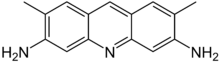
Acridine yellow
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,7-Dimethylacridine-3,6-diamine | |
| Other names
2,7-Dimethylproflavine Acridine yellow G | |
| Identifiers | |
| 92-26-2 | |
| 3D model (Jmol) | Interactive image Interactive image |
| 5-22-11-00340 | |
| ChEBI | CHEBI:248841 |
| ChEMBL | ChEMBL329221 |
| ChemSpider | 8348 |
| ECHA InfoCard | 100.001.947 |
| EC Number | 202-141-5 |
| MeSH | Acridine+yellow |
| PubChem | 8672 |
| RTECS number | AR8790000 |
| |
| |
| Properties | |
| C15H15N3 | |
| Molar mass | 273.30 g/mol |
| Appearance | Brown/red crystals |
| Hazards | |
| EU classification (DSD) |
Harmful (XN) |
| R-phrases | R20/21/22, R36/37/38, R68 |
| S-phrases | S26, S36/37/39 |
| R/S statement | R:R1, R2 S:(S1), (S2) |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Acridine yellow, also known as acridine yellow G, acridine yellow H107, basic yellow K, and 3,6-diamino-2,7-dimethylacridine, is a yellow dye with strong bluish-green fluorescence. It is a derivate of acridine. In histology, it is used as a fluorescent stain, and as a fluorescent probe for non-invasive measurements of cytoplasmic pH changes in whole cells. It is also used as a topical antiseptic. It is usually available as a hydrochloride salt. Acridine yellow damages DNA and is used as a mutagen in microbiology.
Acridine yellow is similar to acridine orange.
External links
This article is issued from Wikipedia - version of the 4/12/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
