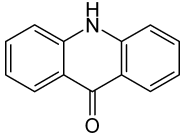
Acridone
 | |
| Names | |
|---|---|
| IUPAC name
10H-acridin-9-one | |
| Identifiers | |
| 578-95-0 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:50756 |
| ChEMBL | ChEMBL436589 |
| ChemSpider | 10188539 |
| ECHA InfoCard | 100.008.578 |
| PubChem | 2015 |
| |
| |
| Properties | |
| C13H9NO | |
| Molar mass | 195.22 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Acridone is an organic compound based on the acridine skeleton, with a carbonyl group at the 9 position. It may be synthesized by the self-condensation of N-phenylanthranilic acid.[1]
Derivatives
Acridone constitutes the scaffold of some synthetic compounds with various pharmacological activities. Most recent derivatives still in its development stage, including 3-chloro-6-(2-diethylamino-ethoxy)-10-(2-diethylamino-ethyl)-acridone, have shown some promise as a potential antimalarial drugs.[2][3]
2-chloroacridone is the precursor used to make clomacran (tranquilizer).
References
- ↑ C. F. H. Allen; G. H. W. McKee (1943). "Acridone". Org. Synth.; Coll. Vol., 2, p. 15
- ↑ HISASHI FUJIOKA; YUKIHIRO NISHIYAMA; HIROSHI FURUKAWA & NOBUO KUMADA (1989). "In Vitro and In Vivo Activities of Atalaphillinine and Related Acridone Alkaloids against Rodent Malaria". Antimicrobial Agents and Chemotherapy. 33 (1): 6–9. doi:10.1128/aac.33.1.6. PMC 171411
 . PMID 2653215.
. PMID 2653215. - ↑ Kelly, Jane X.; Smilkstein, Martin J.; Brun, Reto; Wittlin, Sergio; Cooper, Roland A.; Lane, Kristin D.; Janowsky, Aaron; Johnson, Robert A.; Dodean, Rozalia A.; Winter, Rolf; Hinrichs, David J.; Riscoe, Michael K. (2009). "Discovery of dual function acridones as a new antimalarial chemotype". Nature. 459 (7244): 270–273. doi:10.1038/nature07937. PMID 19357645.
This article is issued from Wikipedia - version of the 5/19/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.