Alkaline hydrolysis
For other uses, see Alkaline hydrolysis (death custom).
Alkaline hydrolysis, in organic chemistry, usually refers to types of nucleophilic substitution reactions in which the attacking nucleophile is a hydroxide ion.
Example
In the alkaline hydrolysis of esters and amides the hydroxide ion nucleophile attacks the carbonyl carbon in a nucleophilic acyl substitution reaction. This mechanism is supported by isotope labeling experiments. For example, when ethyl propionate with an oxygen-18 labeled ethoxy group is treated with sodium hydroxide (NaOH), the oxygen-18 is completely absent from the sodium propionate product and is found exclusively in the ethanol formed.[1]
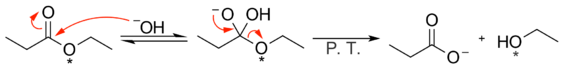
Uses
Drain cleaners take advantage of this method to dissolve hair and fat in pipes.
See also
References
This article is issued from Wikipedia - version of the 5/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.