Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene (or their equivalents).[1] An alkyl group is a piece of a molecule with the general formula CnH2n+1, where n is the integer depicting the number of carbons linked together. For example, a methyl group (n = 1, CH3) is a fragment of a methane molecule (CH4). Alkylating agents utilize selective alkylation by adding the desired aliphatic carbon chain to the previously chosen starting molecule. This is one of many known chemical syntheses. Alkyl groups can also be removed in a process known as dealkylation.
In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces synthetic C7–C8 alkylate, which is a premium blending stock for gasoline.[2]
In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.
Alkylating agents
Alkylating agents are classified according to their nucleophilic or electrophilic character.
Nucleophilic alkylating agents
Nucleophilic alkylating agents deliver the equivalent of an alkyl anion (carbanion). Examples include the use of organometallic compounds such as Grignard (organomagnesium), organolithium, organocopper, and organosodium reagents. These compounds typically can add to an electron-deficient carbon atom such as at a carbonyl group. Nucleophilic alkylating agents can also displace halide substituents on a carbon atom. In the presence of catalysts, they also alkylate alkyl and aryl halides, as exemplified by Suzuki couplings.
Electrophilic alkylating agents
Electrophilic alkylating agents deliver the equivalent of an alkyl cation. Examples include the use of alkyl halides with a Lewis acid catalyst to alkylate aromatic substrates in Friedel-Crafts reactions. Alkyl halides can also react directly with amines to form C-N bonds; the same holds true for other nucleophiles such as alcohols, carboxylic acids, thiols, etc. Trimethyloxonium tetrafluoroborate and triethyloxonium tetrafluoroborate are particularly strong electrophiles due to their overt positive charge and an inert leaving group (dimethyl or diethyl ether).
Electrophilic, soluble alkylating agents are often very toxic, due to their ability to alkylate DNA. They should be handled with proper PPE. This mechanism of toxicity is also responsible for the ability of some alkylating agents to perform as anti-cancer drugs in the form of alkylating antineoplastic agents, and also as chemical weapons such as mustard gas. Alkylated DNA either does not coil or uncoil properly, or cannot be processed by information-decoding enzymes. This results in cytotoxicity with the effects of inhibition the growth of the cell, initiation of programmed cell death or apoptosis. However, mutations are also triggered, including carcinogenic mutations, explaining the higher incidence of cancer after exposure.
Alcohols and phenols can be alkylated to give alkyl ethers:
The produced acid HX is neutralized with a base, or, alternatively, the alcohol is deprotonated first to give an alkoxide or phenoxide. For example, dimethyl sulfate alkylates the sodium salt of phenol to give anisole, the methyl ether of phenol. The dimethyl sulfate is dealkylated to sodium methylsulfate.[3]
- (with Na+ as a spectator ion)
On the contrary, the alkylation of amines introduces the problem that the alkylation of an amine makes it more nucleophilic. Thus, when an electrophilic alkylating agent is introduced to a primary amine, it will preferentially alkylate all the way to a quaternary ammonium cation.
- (alkylating agent omitted for clarity)
If the quaternary ammonium is not the desired product, more circuitous routes such as reductive amination are necessary.
Carbene alkylating agents
Carbenes are extremely reactive and are known to attack even unactivated C-H bonds. Carbenes can be generated by elimination of a diazo group. Unlike electrophilic or nucleophilic alkylating agents, carbenes are neutral, and they insert into bonds rather than discard leaving groups. A metal can form a carbene equivalent called a transition metal carbene complex.
Catalysts
Silicotungstic acid is used to manufacture ethyl acetate by the alkylation of acetic acid by ethylene:
- C2H4 + CH3CO2H → CH3CO2C2H5
It has also been commercialized for the oxidation of ethylene to acetic acid:[4]
- C2H4 + O2 → CH3CO2H
In biology
Methylation is the most common type of alkylation, being associated with the transfer of a methyl group. Methylation is distinct from alkylation in that it is specifically the transfer of one carbon, whereas alkylation can refer to the transfer of long chain carbon groups. Methylation in nature is typically effected by vitamin B12-derived enzymes, where the methyl group is carried by cobalt. In methanogenesis, coenzyme M is methylated by tetrahydromethanopterin.
Electrophilic compounds may alkylate different nucleophiles in the body. The toxicity, carcinogenity, and paradoxically, cancer cell-killing abilities of different DNA alkylating agents are an example.
Demethylation is the reverse of methylation.
Oil refining
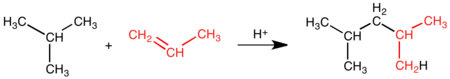
In a standard oil refinery process, isobutane is alkylated with low-molecular-weight alkenes (primarily a mixture of propene and butene) in the presence of a Bronsted acid catalyst, either sulfuric acid or hydrofluoric acid.[5] In an oil refinery it is referred to as a sulfuric acid alkylation unit (SAAU) or a hydrofluoric alkylation unit, (HFAU). Refinery workers may simply refer to it as the alky or alky unit. The catalyst protonates the alkenes (propene, butene) to produce reactive carbocations, which alkylate isobutane. The reaction is carried out at mild temperatures (0 and 30 °C) in a two-phase reaction. Because the reaction is exothermic, cooling is needed: SAAU plants require lower temperatures so the cooling medium needs to be chilled, for HFAU normal refinery cooling water will suffice. It is important to keep a high ratio of isobutane to alkene at the point of reaction to prevent side reactions which produces a lower octane product, so the plants have a high recycle of isobutane back to feed. The phases separate spontaneously, so the acid phase is vigorously mixed with the hydrocarbon phase to create sufficient contact surface.
The product is called alkylate and is composed of a mixture of high-octane, branched-chain paraffinic hydrocarbons (mostly isoheptane and isooctane). Alkylate is a premium gasoline blending stock because it has exceptional antiknock properties and is clean burning. Alkylate is also a key component of avgas. The octane number of the alkylate depends mainly upon the kind of alkenes used and upon operating conditions. For example, isooctane results from combining butylene with isobutane and has an octane rating of 100 by definition. There are other products in the alkylate, so the octane rating will vary accordingly.
Since crude oil generally contains only 10 to 40 percent of hydrocarbon constituents in the gasoline range, refineries use a fluid catalytic cracking process to convert high molecular weight hydrocarbons into smaller and more volatile compounds, which are then converted into liquid gasoline-size hydrocarbons. Alkylation processes transform low molecular-weight alkenes and iso-paraffin molecules into larger iso-paraffins with a high octane number.
Combining cracking, polymerization, and alkylation can result in a gasoline yield representing 70 percent of the starting crude oil. More advanced processes, such as cyclicization of paraffins and dehydrogenation of naphthenes forming aromatic hydrocarbons in a catalytic reformer, have also been developed to increase the octane rating of gasoline. Modern refinery operation can be shifted to produce almost any fuel type with specified performance criteria from a single crude feedstock.
Refineries examine whether it makes sense economically to install alkylation units. Alkylation units are complex, with substantial economy of scale. In addition to a suitable quantity of feedstock, the price spread between the value of alkylate product and alternate feedstock disposition value must be large enough to justify the installation. Alternative outlets for refinery alklylation feedstocks include sales as LPG, blending of C4 streams directly into gasoline to lower the flash point of the product and feedstocks for chemical plants. Local market conditions vary widely between plants. Variation in the RVP (Reid vapor pressure) specification for gasoline between countries and between seasons dramatically impacts the amount of butane streams that can be blended directly into gasoline. The transportation of specific types of LPG streams can be expensive so local disparities in economic conditions are often not fully mitigated by cross market movements of alkylation feedstocks.
The availability of a suitable catalyst is also an important factor in deciding whether to build an alkylation plant. If sulfuric acid is used, significant volumes are needed. Access to a suitable plant is required for the supply of fresh acid and the disposition of spent acid. If a sulfuric acid plant must be constructed specifically to support an alkylation unit, such construction will have a significant impact on both the initial requirements for capital and ongoing costs of operation. Alternatively it is possible to install a WSA Process unit to regenerate the spent acid. No drying of the gas takes place. This means that there will be no loss of acid, no acidic waste material and no heat is lost in process gas reheating. The selective condensation in the WSA condenser ensures that the regenerated fresh acid will be 98% w/w even with the humid process gas. It is possible to combine spent acid regeneration with disposal of hydrogen sulfide by using the hydrogen sulfide as internal fuel in the refinery or elsewhere.[6]
The second main catalyst option is hydrofluoric acid. In typical alkylation plants, rates of consumption for acid are much lower than for sulfuric acid. These plants also produce alkylate with better octane rating than do sulfuric plants. However, due to its hazardous nature, HF acid is produced at very few locations and transportation must be managed rigorously.
See also
- Hydrodealkylation
- Transalkylation
- Alkynation
- Friedel–Crafts reaction
- Category:Alkylating agents
- Category:Ethylating agents
- Category:Methylating agents
References
- ↑ March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. ISBN 0-471-85472-7
- ↑ Stefanidakis, G.; Gwyn, J.E. (1993). "Alkylation". In John J. McKetta. Chemical Processing Handbook. CRC Press. pp. 80–138. ISBN 0-8247-8701-3.
- ↑ G. S. Hiers and F. D. Hager (1941). "Anisole". Org. Synth.; Coll. Vol., 1, p. 58
- ↑ Misono, Makoto (2009). "Recent progress in the practical applications of heteropolyacid and perovskite catalysts: Catalytic technology for the sustainable society". Catalysis Today. 144 (3-4): 285–291. doi:10.1016/j.cattod.2008.10.054.
- ↑ Michael Röper, Eugen Gehrer, Thomas Narbeshuber, Wolfgang Siegel "Acylation and Alkylation" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000. doi:10.1002/14356007.a01_185
- ↑ Sulphur recovery; (2007). The Process Principles, details advances in sulphur recovery by the WSA process. Denmark: Jens Kristen Laursen, Haldor Topsøe A/S. Reprinted from Hydrocarbonengineering August 2007
External links
- Macrogalleria page on polycarbonate production
- Alkylating agents at the US National Library of Medicine Medical Subject Headings (MeSH)
