Aluminium iodide
 | |
| | |
| Names | |
|---|---|
| Preferred IUPAC name
Aluminium iodide | |
| Other names
Aluminium(III) iodide Aluminum iodide | |
| Identifiers | |
| 7784-23-8 (anhydrate) 10090-53-6 (hexahydrate) | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 74202 (anhydrate) |
| ECHA InfoCard | 100.029.140 |
| EC Number | 232-054-8 |
| PubChem | 82222 (anhydrate) |
| UN number | UN 3260 |
| |
| |
| Properties | |
| AlI3 | |
| Molar mass | 407.69495 g/mol (anhydrous) 515.786 g/mol (hexahydrate) |
| Appearance | white powder but impure samples are often brown |
| Density | 3.98 g/cm3 (anhydrous) 2.63 g/cm3 (hexahydrate) |
| Melting point | 189.4 °C (372.9 °F; 462.5 K) (anhydrous) 185 °C, decomposes (hexahydrate) |
| Boiling point | 360 °C (680 °F; 633 K) , sublimes |
| reacts violently (anhydrous) soluble (hexahydrate) | |
| Solubility in alcohol, ether | soluble (hexahydrate) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Aluminium iodide is any chemical compound containing only aluminium and iodine. Invariably, the name refers to a compound of the composition , formed by the reaction of aluminium and iodine[1] or the action of HI on Al metal. The hexahydrate is obtained from a reaction between metallic aluminum or aluminum hydroxide with hydrogen iodide or hydroiodic acid. Like the related chloride and bromide, is a strong Lewis acid and will absorb water from the atmosphere. It is employed as a reagent for the scission of certain kinds of C-O and N-O bonds. It cleaves aryl ethers and deoxygenates epoxides.[2]
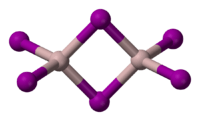
Structure
Solid is dimeric, consisting of , similar to that of AlBr3.[3] The structure of monomeric and dimeric forms have been characterized in the gas phase.[4] The monomer, is trigonal planar with a bond length of 2.448(6) Å, and the bridged dimer, at 430 K is a similar to Al2Cl6 and Al2Br6 with bond lengths of 2.456(6) Å (terminal) and 2.670(8) Å (bridging). The dimer is described as floppy with an equilibrium geometry of D2h.
Aluminium(I) iodide

Planned and performed by Marina Stojanovska, Miha Bukleski and Vladimir Petruševski, Department of Chemistry, FNSM, Ss. Cyril and Methodius University, Skopje, Macedonia.
The name "aluminium iodide" is widely assumed to describe the triiodide or its dimer. In fact, a monoiodide also enjoys a role in the system, although the compound AlI is unstable at room temperature relative to the triiodide[5]
An illustrative derivative of aluminium monoiodide is the cyclic adduct formed with triethylamine, .
References
- ↑ G. W. Watt; J. L. Hall (1953). Inorganic Syntheses. IV. pp. 117–119.
- ↑ M. Gugelchuk (2004). Aluminum Iodide, in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette). New York: J. Wiley & Sons. doi:10.1002/047084289X.ra083.
- ↑ Troyanov, Sergey I.; Krahl, Thoralf; Kemnitz, Erhard (2004). "Crystal structures of GaX3(X= Cl, Br, I) and AlI3". Zeitschrift für Kristallographie. 219 (2-2004): 88–92. doi:10.1524/zkri.219.2.88.26320. ISSN 0044-2968.
- ↑ Hargittai, Magdolna; Réffy, Balázs; Kolonits, Mária (2006). "An Intricate Molecule: Aluminum Triiodide. Molecular Structure of AlI3and Al2I6from Electron Diffraction and Computation". The Journal of Physical Chemistry A. 110 (10): 3770–3777. doi:10.1021/jp056498e. ISSN 1089-5639.
- ↑ Dohmeier, C.; Loos, D.; Schnöckel, H. (1996). "Aluminum(I) and Gallium(I) Compounds: Syntheses, Structures, and Reactions". Angewandte Chemie International Edition. 35: 129–149. doi:10.1002/anie.199601291.