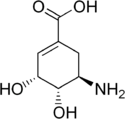
Aminoshikimic acid
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(3R,4S,5R)-5-Amino-3,4-dihydroxycyclohex-1-enecarboxylic acid | |||
| Other names
Aminoshikimate | |||
| Identifiers | |||
| 178948-66-8 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 391778 | ||
| PubChem | 443636 | ||
| |||
| |||
| Properties | |||
| C7H11NO4 | |||
| Molar mass | 173.17 g·mol−1 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Aminoshikimic acid is a synthetic crystalline carboxylic acid. It is characterized by multiple stereogenic centers and functional groups arrayed around a six-membered carbocyclic ring. Aminoshikimic acid is also an alternative to shikimic acid as a starting material for the synthesis of neuraminidase inhibitors such as the antiinfluenza agent oseltamivir (Tamiflu).
History
Aminoshikimic acid is an unnatural carbohydrate, although aminoshikimic acid is the namesake of the aminoshikimate pathway, which generates the 3-amino-5-hydroxybenzoic acid (AHBA) starter unit required for the biosynthesis of the ansamycins and mitomycins.[1] The first microbe-catalyzed syntheses of aminoshikimic acid were described by Guo and Frost in 2004.[2]
Pharmaceutical uses
Aminoshikimic acid is an intriguing alternative to shikimic acid as a starting material for the synthesis of neuraminidase inhibitors such as the antiinfluenza agent oseltamivir.[3] Aminoshikimic acid is also a versatile chiral starting material for the synthesis of new pharmaceuticals. As with shikimic acid, aminoshikimic acid is an attractive candidate for use as the core scaffold for synthesis of combinatorial libraries.
References
- ↑ Floss, H. G. (1997). "Natural products derived from unusual variants of the shikimate pathway.". Nat Prod Rep. 14 (5): 433–452. doi:10.1039/np9971400433. PMID 9364776.
- ↑ Guo, Jiantao; Frost, John (2004). "Synthesis of Aminoshikimic Acid". Org. Lett. 6 (10): 1585–1588. doi:10.1021/ol049666e. PMID 15128242.
- ↑ Frost, J.; Guo, J. (2007). "Synthesis of oseltamivir carboxylates, US patent 2007/0190621 A1".