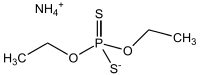
Ammonium diethyl dithiophosphate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Azanium; diethoxy-sulfanylidene-sulfido-λ5-phosphane | |
| Other names
Ammonium O,O′-diethyldithiophosphate; Ammonium O,O-diethyl phosphorodithioate; Ammonium O,O-diethyl dithiophosphate; Ammonium O,O-diethyl diethiophosphate; Phosphorodithioic acid, O,O-diethyl ester, ammonium salt | |
| Identifiers | |
| 1068-22-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 13417 |
| ECHA InfoCard | 100.012.676 |
| PubChem | 14036 |
| UNII | NSX3KEL604 |
| |
| |
| Properties | |
| C4H14NO2PS2 | |
| Molar mass | 203.25 g·mol−1 |
| Appearance | White to yellowish crystals |
| Melting point | 438 K (165 °C) |
| Hazards | |
| EU classification (DSD) |
Harmful (Xn) |
| R-phrases | R20/21/22 |
| S-phrases | S36 |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ammonium diethyl dithiophosphate or more systematically ammonium O,O′-diethyl dithiophosphate, is the ammonium salt of diethyl dithiophosphoric acid. It is used as a source of the (C2H5O)2PS2− ligand in coordination chemistry and in analytical chemistry for determination of various ions. It can be obtained by the reaction of phosphorus pentasulfide with ethanol and ammonia. In crystal structure of this compound the ammonium cation is connected by four charge-assisted N—H···S hydrogen bonds to four tetrahedral diethyl dithiophosphate anions.[1]
References
- ↑ Okuniewski, Andrzej; Becker, Barbara (2011). "Ammonium O,O′-diethyl dithiophosphate". Acta Crystallogr. E. 67 (7): o1749–o1750. doi:10.1107/S1600536811022811.
This article is issued from Wikipedia - version of the 6/30/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
