Ampakine
Ampakines are a class of compounds that take their name from the glutamatergic AMPA receptor with which they strongly interact. The AMPA receptor, in turn, gets its name from AMPA, which selectively binds to it.
They are currently being investigated as potential treatment for a range of conditions involving mental disability and disturbances such as Alzheimer's disease, Parkinson's disease, schizophrenia, treatment-resistant depression (TRD) or neurological disorders such as Attention Deficit Hyperactivity Disorder (ADHD), among others.
More recently developed ampakine compounds are much more potent and selective for the AMPA receptor target, and while none of the newer selective ampakine compounds have yet come onto the market, one compound CX717 is currently in Phase II clinical trials as of 2008.
Examples and structure
Five structural classes of ampakine drugs have been developed so far:[1]
- the pyrrolidine derivative racetam drugs such as piracetam and aniracetam
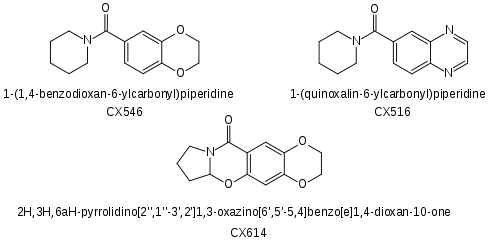
- the CX- series of drugs which encompass a range of benzoylpiperidine and benzoylpyrrolidine structures
- benzothiazide derivatives such as cyclothiazide and IDRA-21
- biarylpropylsulfonamides such as LY-392,098, LY-404,187, LY-451,646, LY-503,430
- benzylpiperazine derivatives
Racetam family
The parent compound in which the AMPA modulating activity was first characterised was the well known nootropic drug aniracetam. Several drugs in the racetam family have been reported as producing ampakine effects, but while this has been well established for some compounds such as aniracetam and pramiracetam, it is unclear if all of the racetam family act in this way, as the racetam drugs appear to have multiple modes of action.
Cortex Pharmaceuticals
Since the discovery of the ampakine mode of action as one of the means by which the racetams produce their nootropic effects, a wide range of more selective ampakine drugs have been developed by Cortex Pharmaceuticals, which hold patents covering most medical uses of this class of drugs. The best known compounds that have come out of the Cortex drug development program are CX-516 (Ampalex), CX-546, CX-614, CX-691 (Farampator) and CX-717. Org 26576 was invented by Cortex but then licensed to Organon for development.
Several other compounds such as CX-701, CX-1739, CX-1763 and CX-1837 have also been announced as being under current investigation, and while little information has yet been released about them, CX-1739 is believed to be the most potent compound in this class to date, reportedly some 5x the potency of CX-717.
Eli Lilly/other
Other compounds producing the ampakine activity profile such as IDRA-21, S-18986, and Eli Lilly's LY-503,430 have been developed by other pharmaceutical companies, but these are only used in animal research at present, and Cortex is the only company currently developing selective ampakine drugs for human use, in partnership with the larger pharmaceutical company Schering-Plough.
Mechanism
Ampakines work by allosterically binding to a type of glutamate receptor in the brain, called AMPA receptors.
Side effects
Few side effects have been determined, but an ampakine called farampator (CX-691) has side effects including headache, somnolence, nausea, and impaired episodic memory.[2]
Uses
An ampakine called CX456 has been proposed as a treatment for Rett syndrome, after favorable testing in an animal model.[3]
Ampakines have been investigated by DARPA for potential use in increasing military effectiveness.[4]
References
- ↑ O'Neill, M. J.; Bleakman, D.; Zimmerman, D. M.; Nisenbaum, E. S. (2004). "AMPA Receptor Potentiators for the Treatment of CNS Disorders". Current Drug Targets. CNS and Neurological Disorders. 3 (3): 181–194. doi:10.2174/1568007043337508. PMID 15180479.
- ↑ Wezenberg, E.; Verkes, R. J.; Ruigt, G. S.; Hulstijn, W.; et al. (2007). "Acute Effects of the Ampakine Farampator on Memory and Information Processing in Healthy Elderly Volunteers" (pdf). Neuropsychopharmacology. 32 (6): 1272–1283. doi:10.1038/sj.npp.1301257. PMID 17119538.
- ↑ Ogier, M.; Wang, H.; Hong, E.; Wang, Q.; et al. (2007). "Brain-derived Neurotrophic Factor Expression and Respiratory Function Improve after Ampakine Treatment in a Mouse Model of Rett Syndrome" (pdf). Journal of Neuroscience. 27 (40): 10912–10917. doi:10.1523/JNEUROSCI.1869-07.2007. PMID 17913925.
- ↑ Saletan, William (2008-07-16). "Night of the Living Meds: The U.S. military's sleep-reduction program". Slate. Retrieved 2012-04-05.
Further reading
- Staubli, U.; Rogers, G.; Lynch, G. (1994). "Facilitation of glutamate receptors enhances memory" (pdf). Proc Natl Acad Sci U S A. 91 (2): 777–781. doi:10.1073/pnas.91.2.777. PMC 43032
 . PMID 8290599.
. PMID 8290599. - Staubli, U.; Perez, Y.; Xu, F. B.; Rogers, G.; et al. (1994). "Centrally active modulators of glutamate receptors facilitate the induction of long-term potentiation in vivo" (pdf). Proc Natl Acad Sci U S A. 91 (23): 11158–11162. doi:10.1073/pnas.91.23.11158. PMC 45186
 . PMID 7972026.
. PMID 7972026. - Arai, A.; Lynch G. (1992). "Factors regulating the magnitude of long-term potention induced by theta pattern stimulation". Brain Research. 598 (1–2): 173–184. doi:10.1016/0006-8993(92)90181-8. PMID 1486479.
- Arai, A.; Silberg, J.; Kessler, M.; Lynch, G. (1995). "Effect of thiocyanate on AMPA receptor mediated responses in excised patches and hippocampal slices". Neuroscience. 66 (4): 815–827. doi:10.1016/0306-4522(94)00616-D. PMID 7544449.
- Suppiramaniam, V.; Bahr, B. A.; Sinnarajah, S.; Owens, K.; et al. (2001). "Member of the Ampakine class of memory enhancers prolongs the single channel open time of reconstituted AMPA receptors". Synapse. 40 (2): 154–158. doi:10.1002/syn.1037. PMID 11252027.
- Porrino, L. J.; Daunais, J. B.; Rogers, G. A.; Hampson, R. E.; et al. (2005). "Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates" (pdf). PLoS Biology. 3 (9): e299. doi:10.1371/journal.pbio.0030299. PMC 1188239
 . PMID 16104830.
. PMID 16104830. - Bast, T.; da Silva, B. M.; Morris, R. G. (2005). "Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory" (pdf). Journal of Neuroscience. 25 (25): 5845–5856. doi:10.1523/JNEUROSCI.0698-05.2005. PMID 15976073.
External links
- Ampakine Article Abstracts
- Recent Article About CX717
- Article on Ampakines with reference to Alzheimers
- US Patent 5,650,409
- US Patent 6,030,968
- US Patent 6,730,677
- US Patent 7,307,073