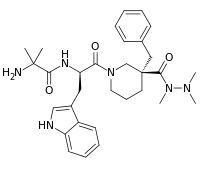
Anamorelin
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | None |
| Pharmacokinetic data | |
| Biological half-life | 6–7 hours[1] |
| Identifiers | |
| |
| CAS Number | 249921-19-5 |
| PubChem (CID) | 9828911 |
| ChemSpider | 8004650 |
| Chemical and physical data | |
| Formula | C31H42N6O3 |
| Molar mass | 546.70358 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Anamorelin (INN) (developmental code names ONO-7643, RC-1291, ST-1291), also known as anamorelin hydrochloride (USAN, JAN), is a non-peptide, orally-active, centrally-penetrant, selective agonist of the ghrelin/growth hormone secretagogue receptor (GHSR) with appetite-enhancing and anabolic effects which is under development by Helsinn Therapeutics for the treatment of cancer cachexia and anorexia.[2][3][4]
Anamorelin significantly increases plasma levels of growth hormone (GH), insulin-like growth factor 1 (IGF-1), and insulin-like growth factor-binding protein 3 (IGFBP-3) in humans, without affecting plasma levels of prolactin, cortisol, insulin, glucose, adrenocorticotropic hormone (ACTH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), or thyroid-stimulating hormone (TSH).[3][5] In addition, anamorelin significantly increases appetite, overall body weight, lean body mass, and muscle strength,[4][5] with increases in body weight correlating directly with increases in plasma IGF-1 levels.[3]
As of February 2016, anamorelin has completed phase III clinical trials for the treatment of cancer cachexia and anorexia associated with non-small-cell lung carcinoma.[6][7] Results of the studies were positive, and the drug is now in preregistration with the European Medicines Agency.
See also
- Capromorelin
- Examorelin (hexarelin)
- GHRP-6 (SKF-110679)
- Ibutamoren (MK-677)
- Ipamorelin
- Macimorelin
- Pralmorelin (GHRP-2)
- Relamorelin
- SM-130,686
- Tabimorelin
References
- ↑ Leese, Philip T.; Trang, John M.; Blum, Robert A.; de Groot, Eleanor (2015). "An open-label clinical trial of the effects of age and gender on the pharmacodynamics, pharmacokinetics and safety of the ghrelin receptor agonist anamorelin". Clinical Pharmacology in Drug Development. 4 (2): 112–120. doi:10.1002/cpdd.175. ISSN 2160-763X.
- ↑ Currow, David C; Abernethy, Amy P (2014). "Anamorelin hydrochloride in the treatment of cancer anorexia-cachexia syndrome". Future Oncology. 10 (5): 789–802. doi:10.2217/fon.14.14. ISSN 1479-6694.
- 1 2 3 Garcia, José M.; Polvino, William J. (2009). "Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers". Growth Hormone & IGF Research. 19 (3): 267–273. doi:10.1016/j.ghir.2008.12.003. ISSN 1096-6374.
- 1 2 Garcia, José M; Boccia, Ralph V; Graham, Charles D; Yan, Ying; Duus, Elizabeth Manning; Allen, Suzan; Friend, John (2015). "Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials". The Lancet Oncology. 16 (1): 108–116. doi:10.1016/S1470-2045(14)71154-4. ISSN 1470-2045.
- 1 2 Garcia, José M.; Friend, John; Allen, Suzan (2012). "Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study". Supportive Care in Cancer. 21 (1): 129–137. doi:10.1007/s00520-012-1500-1. ISSN 0941-4355.
- ↑ Zhang, Hongjie; Garcia, Jose M (2015). "Anamorelin hydrochloride for the treatment of cancer-anorexia-cachexia in NSCLC". Expert Opinion on Pharmacotherapy. 16 (8): 1245–1253. doi:10.1517/14656566.2015.1041500. ISSN 1465-6566.
- ↑ Temel, Jennifer S; Abernethy, Amy P; Currow, David C; Friend, John; Duus, Elizabeth M; Yan, Ying; Fearon, Kenneth C (2016). "Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials". The Lancet Oncology. 17 (4): 519–531. doi:10.1016/S1470-2045(15)00558-6. ISSN 1470-2045.