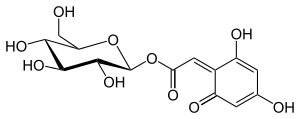
Anthocyanone A
Anthocyanone A
 |
| Names |
| Other names
8-β-d-glucopyranosyl-2,4-dihydroxy-6-oxo-cyclohexa-2,4-dienyl acetic acid |
| Identifiers |
| 3D model (Jmol) |
Interactive image |
| ChemSpider |
29784776 |
InChI=1S/C14H16O10/c15-4-9-11(20)12(21)13(22)14(23-9)24-10(19)3-6-7(17)1-5(16)2-8(6)18/h1-3,9,11-17,20-22H,4H2/b6-3+/t9-,11-,12+,13-,14+/m1/s1 Key: VOWKJMFKRCLSJJ-BFYJNFCASA-N InChI=1/C14H16O10/c15-4-9-11(20)12(21)13(22)14(23-9)24-10(19)3-6-7(17)1-5(16)2-8(6)18/h1-3,9,11-17,20-22H,4H2/b6-3+/t9-,11-,12+,13-,14+/m1/s1 Key: VOWKJMFKRCLSJJ-BFYJNFCABS
|
C1=C(C=C(/C(=C\C(=O)O[C@H]2[C@@H]([C@H]([C@@H]([C@H](O2)CO)O)O)O)/C1=O)O)O
|
| Properties |
| |
C14H16O10 |
| Molar mass |
344.27 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
|
| Infobox references |
|
|
Anthocyanone A is a degradation product of malvidin 3-O-glucoside under acidic conditions.[1] It is found in wine.[2]
References
- ↑ Lopes, P; Richard, T; Saucier, C; Teissedre, PL; Monti, JP; Glories, Y (2007). "Anthocyanone A: A quinone methide derivative resulting from malvidin 3-O-glucoside degradation". Journal of Agricultural and Food Chemistry. 55 (7): 2698–704. doi:10.1021/jf062875o. PMID 17338545.
- ↑ Saucier, Cédric (2010). "How do wine polyphenols evolve during wine ageing?". Cerevisia. 35: 11–15. doi:10.1016/j.cervis.2010.05.002.
|
|---|
|
| 3-hydroxyanthocyanidins | |
|---|
|
| 3-deoxyanthocyanidins | |
|---|
|
| O-methylated anthocyanidins | |
|---|
|
Anthocyanins
(anthocyaninidin glycosides) | Glucosides:
Diglucosides:
- Cyanin (Cyanidin-3,5-O-diglucoside)
- Delphin (Delphinidin-3,5-O-diglucoside)
- Malvin (Malvidin 3,5-diglucoside)
- Pelargonin (Pelargonidin-3,5-O-diglucoside)
- Peonin (Peonidin 3,5-O-diglucoside)
- Petunin (Petunidin 3,5-O-diglucoside)
Others glycosides:
- Antirrhinin (Cyanidin-3-O-rutinoside)
- Ideain (Cyanidin 3-O-galactoside)
- Delphinidin 3-O-rhamnoside
- Petunidin 3-O-arabinoside
- Petunidin 3-O-galactoside
- Petunidin 3-O-rhamnoside
- Petunidin-3-O-rutinoside
- Primulin (Malvidin-3-O-galactoside)
- Pulchellidin 3-rhamnoside
- Tulipanin (Delphinidin 3-O-rutinoside)
|
|---|
|
| Acylated anthocyanins | |
- Cyanidin-3-O-(6-acetyl)-glucoside
- Delphinidin-3-O-(6-acetyl)-glucoside
- Malvidin-3-O-(6-acetyl)-glucoside
- Petunidin-3-O-(6-acetyl)-galactoside
- Petunidin-3-O-(6-acetyl)-glucoside
- Peonidin-3-O-(6-acetyl)-glucoside
|
|---|
| Coumaroylated anthocyanins
(cis- and trans-) | |
|---|
| Caffeoylated anthocyanins |
- Malvidin-3-O-(6-p-caffeoyl)glucoside
- Peonidin-3-O-(6-p-caffeoyl)glucoside
|
|---|
| Malonylated anthocyanins |
- Malonylmalvin (malvidin 3-(6″-malonylglucoside)-5-glucoside)
|
|---|
| Acylated anthocyanin diglycosides |
- Cyanidin-3-O-(di-p-coumarylglucoside)-5-glucoside
- Gentiodelphin (delphinidin 3-O-glucosyl-5-O-(6-O-caffeoyl-glucosyl)-3′-O-(6-O-caffeoyl-glucoside))
- Nasunin (Delphinidin-3-(p-coumaroylrutinoside)-5-glucoside)
- Petanin (petunidin 3-[6-O-(4-O-E-p-coumaroyl-O-α-l-rhamnopyranosyl)-β-d-glucopyranoside]-5-O-β-d-glucopyranoside)
- Violdelphin (Delphinidin 3-rutinoside-7-O-(6-O-(4-(6-O-(4-hydroxybenzoyl)-beta-D-glucosyl)oxybenzoyl)-beta-D-glucoside)
|
|---|
|
|---|
|
| Flavanol-anthocyanin adducts |
- Malvidin glucoside-ethyl-catechin
- Catechin(4α→8)pelargonidin 3-O-β-glucopyranoside
- Epicatechin(4α→8)pelargonidin 3-O-β-glucopyranoside
- Afzelechin(4α→8)pelargonidin 3-O-β-glucopyranoside
- Epiafzelechin(4α→8)pelargonidin 3-O-β-glucopyranoside
|
|---|
|
| Misc. | |
|---|
