Blinatumomab
| Monoclonal antibody | |
|---|---|
| Type | Bi-specific T-cell engager |
| Source | Mouse |
| Target | CD19, CD3 |
| Clinical data | |
| Trade names | Blincyto |
| Pregnancy category |
|
| Routes of administration | intravenous |
| ATC code | L01XC19 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Metabolism | degradation into small peptides and amino acids |
| Biological half-life | 2.11 hours |
| Excretion | urine (negligible) |
| Identifiers | |
| CAS Number |
853426-35-4 |
| ChemSpider | none |
| UNII |
4FR53SIF3A |
| Chemical and physical data | |
| Formula | C2367H3577N649O772S19 |
| Molar mass | 54.1 kg/mol |
| | |
Blinatumomab (trade name Blincyto, previously known as AMG103 and MT103) is a biopharmaceutical drug used as a second-line treatment for Philadelphia chromosome-negative relapsed or refractory acute lymphoblastic leukemia. It belongs to a class of constructed monoclonal antibodies, bi-specific T-cell engagers (BiTEs), that exert action selectively and direct the human immune system to act against tumor cells. Blinatumomab specifically targets the CD19 antigen present on B cells.[1] In December 2014 it was approved by the US Food and Drug Administration under the accelerated approval program; marketing authorization depended on the outcome of clinical trials that were ongoing at the time of approval.[2][3] When it launched, blinatumomab was priced at $178,000 per year in the United States; only about 1,000 people were eligible to take the drug, based on its label.[4]
Medical use
Blinatumomab is used as a second-line treatment for Philadelphia chromosome-negative relapsed or refractory Bcell precursor acute lymphoblastic leukemia.[2]
Mechanism of action
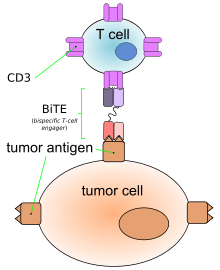
Blinatumomab enables a patient's T cells to recognize malignant B cells. A molecule of blinatumomab combines two binding sites: a CD3 site for T cells and a CD19 site for the target B cells. CD3 is part of the T cell receptor. The drug works by linking these two cell types and activating the T cell to exert cytotoxic activity on the target cell.[5] CD3 and CD19 are expressed in both pediatric and adult patients, making blinatumomab a potential therapeutic option for both pediatric and adult populations.[6]
History
The drug (originally known as MT103) was developed by a German-American company Micromet, Inc. in cooperation with Lonza; In 2012 Micromet was purchased by Amgen, which has furthered the drug's clinical trials.
In July 2014, the FDA granted breakthrough therapy status to blinatumomab for the treatment of acute lymphoblastic leukemia (ALL).[7] In October 2014, Amgen’s Biologics License Application for blinatumomab was granted priority review designation by the FDA, thus establishing a deadline of May 19, 2015 for completion of the FDA review process.[8]
On December 3, 2014, the drug was approved for use in the United States to treat Philadelphia chromosome-negative relapsed or refractory acute lymphoblastic leukemia under the FDA's accelerated approval program; marketing authorization depended on the outcome of clinical trials that were ongoing at the time of approval.[2][9]
Cost
When blinatumomab was approved, Amgen announced that the price for the drug would be $178,000 per year, which made it the most expensive cancer drug on the market. Merck's pembrolizumab was priced at $150,000 per year when it launched (in Sept 2014); unlike that drug and others, only about 1,000 people can be given the drug, based on its label.[4]
Peter Bach, director of the Center for Health Policy and Outcomes at Memorial Sloan-Kettering Cancer Center, has calculated that according to "value-based pricing," assuming that the value of a year of life is $120,000 with a 15% "toxicity discount," the market price of blinaumomab should be $12,612 a month, compared to the market price of $64,260 a month. A representative of Amgen said, “The price of Blincyto reflects the significant clinical, economic and humanistic value of the product to patients and the health-care system. The price also reflects the complexity of developing, manufacturing and reliably supplying innovative biologic medicines.”[10]
External links
References
- ↑ "blinatumomab" (PDF). United States Adopted Names Council » Adopted Names. American Medical Association. 2008. N08/16.(registration required)
- 1 2 3 Blinatumomab label Updated 12/2014
- ↑ Food and Drug Administration December 3, 2014 FDA Press release: Blinatumomab
- 1 2 Tracy Staton for FiercePharmaMarketing. December 18, 2014 Amgen slaps record-breaking $178K price on rare leukemia drug Blincyto
- ↑ Mølhøj, M; Crommer, S; Brischwein, K; Rau, D; Sriskandarajah, M; Hoffmann, P; Kufer, P; Hofmeister, R; Baeuerle, PA (March 2007). "CD19-/CD3-bispecific antibody of the BiTE class is far superior to tandem diabody with respect to redirected tumor cell lysis". Molecular Immunology. 44 (8): 1935–43. doi:10.1016/j.molimm.2006.09.032. PMID 17083975.

- ↑ Amgen (30 October 2012). Background Information for the Pediatric Subcommittee of the Oncologic Drugs Advisory Committee Meeting 04 December 2012 (PDF) (PDF). Food and Drug Administration. Blinatumomab (AMG 103).
- ↑ "Amgen Receives FDA Breakthrough Therapy Designation For Investigational BiTE® Antibody Blinatumomab In Acute Lymphoblastic Leukemia" (Press release). Amgen. 1 July 2014.
- ↑ "Amgen's BiTE® Immunotherapy Blinatumomab Receives FDA Priority Review Designation In Acute Lymphoblastic Leukemia" (Press release). Amgen. 9 October 2014.
- ↑ "Business: Antibody advance". Seven Days. Nature (paper). 516 (7530): 149. 11 December 2014. doi:10.1038/516148a.

- ↑ Peter Loftus (June 18, 2015). "How Much Should Cancer Drugs Cost? Memorial Sloan Kettering doctors create pricing calculator that weighs factors such as side effects, extra years of life". The Wall Street Journal. Retrieved 22 June 2015.