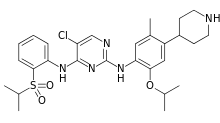
Ceritinib
 | |
| Clinical data | |
|---|---|
| Trade names | Zykadia |
| AHFS/Drugs.com | Multum Consumer Information |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | L01XE28 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | LDK378 |
| CAS Number | 1032900-25-6 |
| PubChem (CID) | 57379345 |
| DrugBank | DB09063 |
| ChemSpider | 29315053 |
| UNII |
K418KG2GET |
| KEGG | D10551 |
| ChEBI | CHEBI:78432 |
| ChEMBL | CHEMBL2403108 |
| Chemical and physical data | |
| Formula | C28H36ClN5O3S |
| Molar mass | 558.14 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Ceritinib (trade name Zykadia) is a drug for the treatment of a specific type of lung cancer.[1] It is an anaplastic lymphoma kinase (ALK) inhibitor.[2] It was approved in April 2014 by the Food and Drug Administration for the treatment of ALK-positive metastatic non-small cell lung cancer (NSCLC) following treatment with crizotinib.[1]
References
- 1 2 "FDA Approves Ceritinib for ALK-Positive Lung Cancer". Medscape. April 29, 2014.
- ↑ Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, Camidge DR, Vansteenkiste J, Sharma S, De Pas T, Riely GJ, Solomon BJ, Wolf J, Thomas M, Schuler M, Liu G, Santoro A, Lau YY, Goldwasser M, Boral AL, Engelman JA (2014). "Ceritinib in ALK-Rearranged Non–Small-Cell Lung Cancer". N Engl J Med. 370: 1189–1197. doi:10.1056/NEJMoa1311107. PMID 24670165.
This article is issued from Wikipedia - version of the 8/3/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.