Chebulic acid
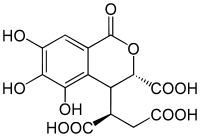 Chebulic acid, according to Lee, 2010.[1] | |
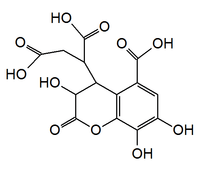 Chebulic acid, according to Klika, 2004.[2] | |
| Names | |
|---|---|
| IUPAC name
(2R)-2-[(3S)-3-carboxy-5,6,7-trihydroxy-1-oxo-3,4-ihydroisochromen-4-yl]butanedioic acid | |
| Other names
Chebuloyl | |
| Identifiers | |
| 23725-05-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 27470963 |
| PubChem | 25255065 |
| |
| |
| Properties | |
| C14H12O11 | |
| Molar mass | 356.23 g/mol |
| Appearance | Brown powder |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Chebulic acid is a phenolic compound isolated from the ripe fruits of Terminalia chebula.[3]
This compound possesses an isomer, neochebulic acid.
Chebulic acid is a component of transformed ellagitannins such as chebulagic acid or chebulinic acid.
References
- ↑ Lee, H. S.; Koo, Y. C.; Suh, H. J.; Kim, K. Y.; Lee, K. W. (2010). "Preventive effects of chebulic acid isolated from Terminalia chebula on advanced glycation endproduct-induced endothelial cell dysfunction". Journal of Ethnopharmacology. 131 (3): 567–574. doi:10.1016/j.jep.2010.07.039. PMID 20659546.
- ↑ The structural and conformational analyses and antioxidant activities of chebulinic acid and its thrice-hydrolyzed derivative, 2,4-chebuloyl-β-D-glucopyranoside, isolated from the fruit of Terminalia chebula. Karel D. Klika, Ammar Saleem, Jari Sinkkonen, Marja Kähkönen, Jyrki Loponen, Petri Tähtinen and Kalevi Pihlaja, ARKIVOC 2004 (vii) 83-105
- ↑ Lee, H. S.; Jung, S. H.; Yun, B. S.; Lee, K. W. (2006). "Isolation of chebulic acid from Terminalia chebula Retz. And its antioxidant effect in isolated rat hepatocytes". Archives of Toxicology. 81 (3): 211–218. doi:10.1007/s00204-006-0139-4. PMID 16932919.
This article is issued from Wikipedia - version of the 11/1/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.