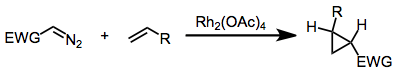Cyclopropanation
Cyclopropanation refers to any chemical process which generates cyclopropane rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroids and a number of quinolone based antibiotics (Ciprofloxacin, Sparfloxacin, etc.). However the high ring strain present in cyclopropanes makes them challenging to produce and generally requires the use of highly reactive species, such as carbenes, ylids and carbanions.[1] Many of the reactions proceed in a cheletropic manner.
Approaches
From alkenes using carbenoid reagents
Several methods exist for converting alkenes to cyclopropane rings using carbene type reagents. As carbene's themselves are highly reactive it is common for them to be used in a stabilised form, referred to as a carbenoid.[2]
Simmons–Smith reaction
In the Simmons–Smith reaction the reactive carbenoid is iodomethylzinc iodide, which is typically formed by a reaction between diiodomethane and a zinc-copper couple. This approach avoids the limitations and safety concerns associated with diazo compounds, which are commonly used in other carbenoid process. However, a major drawback is the high cost of diiodomethane. Modifications involving cheaper alternatives have been developed, such as dibromomethane[3] or diazomethane and zinc iodide.[4] The reactivity of the system can also be increased by exchanging the zinc‑copper couple for diethylzinc,[5] however as this reagent is pyrophoric it must be handled carefully.
Using diazo compounds
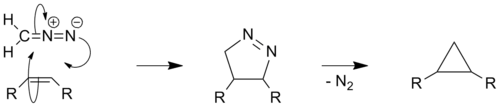
Certain diazo compounds, such as diazomethane, can react with olefins to produce cyclopropanes in a 2 step manner. The first step involves a 1,3-dipolar cycloaddition to form a pyrazoline which then undergoes denitrogenation, either photochemically or by thermal decomposition, to give cyclopropane. The thermal route, which often uses KOH and platinum as catalysts, is also known as the Kishner cyclopropane synthesis after the Russian chemist Nikolai Kischner[6][7] and can also be performed using hydrazine and α,β-unsaturated carbonyl compounds.[8] The mechanism of decomposition has been the subject of several studies and remains somewhat controversial, although it is broadly thought to proceed via a diradical species.[9][10] In terms of green chemistry this method is superior to other carbene based cyclopropanations; as it does not involve metals or halogenated reagents, and produces only N2 as a by-product. However the reaction can be dangerous as trace amounts of unreacted diazo compounds may explode during the thermal rearrangement of the pyrazoline.
Using diazo compounds with metal catalysis
Diazo compounds may be used more safely by reacting them with transition metals compounds (typically containing Cu, Pd, Ni, Co or Rh) to form metal carbenoid complexes. These readily undergo intermolecular metal-catalyzed carbenoid cyclopropanations with olefins[11] and are far safer than the starting diazo materials as they are not explosive. This process also allows for enantioselective synthesis through the presence of chiral ligands or by adding chiral auxiliaries[12] to the diazo compound.
Using free carbenes
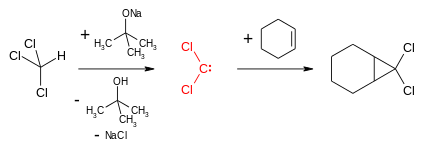
Free carbenes can be employed for cyclopropanation reactions, however there is limited scope for this as few can be produced conveniently and nearly all are unstable (see: carbene dimerization). An exception are dihalocarbene's such as dichlorocarbene or difluorocarbene, which are reasonably stable and will react to form geminal dihalo-cyclopropanes.[13] These compounds can then be used to form allenes via the Skattebøl rearrangement.
The Buchner ring expansion reaction also involves the formation of a stabilised carbene.
From alkenes using ylids
Cyclopropanes can be generated using a sulphur ylide in the Johnson–Corey–Chaykovsky reaction,[14] however this process is largely limited to use on electron-poor olefines, particularly α,β-unsaturated carbonyl compounds.
Intramolecular cyclisation
Cyclopropanes can be obtained by a variety of intramolecular cyclisation reactions. A simple method is to use primary haloalkanes bearing appropriately placed electron withdrawing groups. Treatment with a strong base will generate a carbanion which will cyclise in a 3-exo-trig manner, with displacement of the halide. Examples include the formation of cyclopropyl cyanide[15] and cyclopropylacetylene[16] This mechanism also forms the basis of the Favorskii rearrangement.
A related process is the cyclisation of 1,3-dibromopropane via a Wurtz coupling. This was used for the first synthesis of cyclopropane by August Freund in 1881. Originally this reaction was performed using sodium,[17] however the yield can be improved by exchanging this for zinc.[18]
- BrCH2CH2CH2Br + 2 Na → (CH2)3 + 2 NaBr
Other approaches
- The Kulinkovich reaction form cyclopropanols via a reaction between esters and Grignard reagents in presence of a titanium alkoxide.
- The Bingel reaction is a specialised cyclopropanation reaction used to functionalise a fullerene.
- In the di-pi-methane rearrangement photochemical stimulation causes 1,4-dienes to rearrange to form vinylcyclopropanes.[19] These can then undergo vinylcyclopropane rearrangements
- Cyclopropane-fatty-acyl-phospholipid synthase performs cyclopropanation is biological systems
Biosynthesis
Although cyclopropanes are relatively rare in biochemistry, many cyclopropanation pathways have been identified in nature. The most common pathways involve ring closure reactions of carbocations in terpenoids. Cyclopropane fatty acids are derived from the attack of S-adenosylmethionine (SAM) on unsaturated fatty acids. The precursor to the hormone ethylene, 1-aminocyclopropane-1-carboxylic acid is derived directly from SMM via intramolecular nucleophilic displacement of the SMe2 group subsequent to condensation with pyridoxal phosphate.[20] Direct carbene transfer from diazoesters to olefins has also been achieved through in vitro biocatalysis using engineered variants of the cytochrome P450 enzyme from Bacillus megaterium that were optimized by directed evolution.[21]
Enantioselective and stereoselective synthesis
While cyclopropane is achiral, substituted cyclopropanes very often display chirality. The presence of the cyclopropane motif in a number of drug molecules has made the development of enantioselective synthesis important. In general the chirality of metal catalysed intermolecular processes can be controlled by use of chiral ligands[22] or chiral auxiliaries.[12] other processes may be influenced by asymmetric induction; this area has been reviewed.[23]
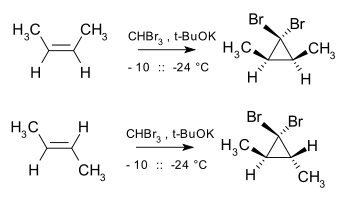
Cyclopropanation is also stereospecific as the addition of carbene and carbenoids to alkenes is a form of a cheletropic reaction, with the addition taking place in a syn manner. For example, dibromocarbene and cis-2-butene yield cis-2,3-dimethyl-1,1-dibromocyclopropane, whereas the trans isomer exclusively yields the trans cyclopropane.[24]
Reactions of cyclopropane rings
Electrophilic addition
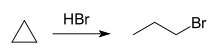
Cyclopropanes undergo hydrohalogenation in the presence of mineral acids to give linear alkyl halides. These reactions follow Markovnikov's rule.[25]
Ring expansion
Cyclopropyl groups adjacent to vinyl groups can undergo ring expansion reactions. Examples include the vinylcyclopropane rearrangement and the divinylcyclopropane-cycloheptadiene rearrangement. This reactivity can be exploited to generate unusual cyclic compounds, such as cyclobutenes,[26] or bicyclic species such as the cycloheptene shown below.[27]
References
- ↑ Pellissier, Hélène (July 2008). "Recent developments in asymmetric cyclopropanation". Tetrahedron. 64 (30-31): 7041–7095. doi:10.1016/j.tet.2008.04.079.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "carbenoids".
- ↑ Fabisch, Bodo; Mitchell, Terence N. (1984). "An inexpensive modification of the Simmons-Smith reaction: The formation of bromomethylzinc bromide as studied by NMR spectroscopy". Journal of Organometallic Chemistry. 269 (3): 219–221. doi:10.1016/0022-328X(84)80305-8.
- ↑ Wittig, Georg; Wingler, Frank (1 August 1964). "Über methylenierte Metallhalogenide, IV. Cyclopropan-Bildung aus Olefinen mit Bis-halogenmethyl-zink". Chemische Berichte. 97 (8): 2146–2164. doi:10.1002/cber.19640970808.
- ↑ Furukawa, J.; Kawabata, N.; Nishimura, J. (1968). "Synthesis of cyclopropanes by the reaction of olefins with dialkylzinc and methylene iodide". Tetrahedron. 24 (1): 53–58. doi:10.1016/0040-4020(68)89007-6.
- ↑ Lewis, David E. (4 November 2013). "Disability, Despotism, Deoxygenation-From Exile to Academy Member: Nikolai Matveevich Kizhner". Angewandte Chemie International Edition. 52 (45): 11704–11712. doi:10.1002/anie.201303165.
- ↑ N. M. Kishner, A. Zavadovskii, J. Russ. Phys. Chem. Soc. 43, 1132 (1911).
- ↑ J. Petersen, R.; P. S. Skell, P. (1967). "PHENYLCYCLOPROPANE". Org. Synth. 47: 98. doi:10.15227/orgsyn.047.0098.
- ↑ Crawford, Robert J.; Mishra, Anupama (September 1966). "The Mechanism of the Thermal Decomposition of 1-Pyrazolines and Its Relationship to Cyclopropane Isomerizations". Journal of the American Chemical Society. 88 (17): 3963–3969. doi:10.1021/ja00969a014.
- ↑ Muray, Elena; Illa, Ona; Castillo, José A.; Álvarez-Larena, Ángel; Bourdelande, José L.; Branchadell, Vicenç; Ortuño, Rosa M. (June 2003). "Photolysis of Chiral 1-Pyrazolines to Cyclopropanes: Mechanism and Stereospecificity". The Journal of Organic Chemistry. 68 (12): 4906–4911. doi:10.1021/jo0342471.
- ↑ Brookhart, Maurice.; Studabaker, William B. (1987). "Cyclopropanes from reactions of transition metal carbene complexes with olefins". Chemical Reviews. 87 (2): 411–432. doi:10.1021/cr00078a008.
- 1 2 Davies, Huw M. L.; Huby, Nicholas J. S.; Cantrell, William R.; Olive, Jennifer L. (October 1993). ".alpha.-Hydroxy esters as chiral auxiliaries in asymmetric cyclopropanations by rhodium(II)-stabilized vinylcarbenoids". Journal of the American Chemical Society. 115 (21): 9468–9479. doi:10.1021/ja00074a012.
- ↑ Fedoryński, Michał (1 April 2003). "Syntheses of Dihalocyclopropanes and Their Use in Organic Synthesis". Chemical Reviews. 103 (4): 1099–1132. doi:10.1021/cr0100087. PMID 12683778.
- ↑ Li, A.-H.; Dai, L.-X.; Aggarwal, V. K. (1997). "Asymmetric Ylide Reactions: Epoxidation, Cyclopropanation, Aziridination, Olefination, and Rearrangement". Chemical Reviews. 97 (6): 2341. doi:10.1021/cr960411r.
- ↑ Schlatter, M. J. (1943). "CYCLOPROPYL CYANIDE". Org. Synth. 23: 20. doi:10.15227/orgsyn.023.0020..
- ↑ Huntington, Martha; Edward G. Corley; Andrew S. Thompson (2000). "CYCLOPROPYLACETYLENE". Org. Synth. 77: 231. doi:10.15227/orgsyn.077.0231.
- ↑ August Freund (1881). "Über Trimethylen". Journal für Praktische Chemie. 26 (1): 625–635. doi:10.1002/prac.18820260125.
- ↑ G. Gustavson (1887). "Ueber eine neue Darstellungsmethode des Trimethylens". J. Prakt. Chem. 36: 300–305. doi:10.1002/prac.18870360127.
- ↑ IUPAC Gold book definition
- ↑ Wessjohann, Ludger A.; Brandt, Wolfgang; Thiemann, Thies (April 2003). "Biosynthesis and Metabolism of Cyclopropane Rings in Natural Compounds". Chemical Reviews. 103 (4): 1625–1648. doi:10.1021/cr0100188.
- ↑ Coelho, P. S.; Brustad, E. M.; Kannan, A.; Arnold, F. H. (20 December 2012). "Olefin Cyclopropanation via Carbene Transfer Catalyzed by Engineered Cytochrome P450 Enzymes". Science. 339 (6117): 307–310. doi:10.1126/science.1231434.
- ↑ Charette, A. B.; Beauchemin, A. Org. React. 2001, 58, 1. doi:10.1002/0471264180.or058.01
- ↑ Lebel, Hélène; Marcoux, Jean-François; Molinaro, Carmela; Charette, André B. (1 April 2003). "Stereoselective Cyclopropanation Reactions". Chemical Reviews. 103 (4): 977–1050. doi:10.1021/cr010007e. PMID 12683775.
- ↑ Skell, P.S.; Garner, A.Y. (1956). "The Stereochemistry of Carbene-Olefin Reactions. Reactions of Dibromocarbene with the cis- and trans-2-Butenes". Journal of the American Chemical Society. 78 (14): 3409–3411. doi:10.1021/ja01595a040.
- ↑ March, Jerry (1985). Advanced organic chemistry : reactions, mechanisms, and structure (3. ed.). New York [u.a.]: Wiley. ISBN 0-471-85472-7.
- ↑ Fürstner, Alois; Aïssa, Christophe (2006). "PtCl-Catalyzed Rearrangement of Methylenecyclopropanes". Journal of the American Chemical Society. 128 (19): 6306–6307. doi:10.1021/ja061392y. PMID 16683781.
- ↑ Wender, Paul A.; Haustedt, Lars O.; Lim, Jaehong; Love, Jennifer A.; Williams, Travis J.; Yoon, Joo-Yong (May 2006). "Asymmetric Catalysis of the [5 + 2] Cycloaddition Reaction of Vinylcyclopropanes and π-Systems". Journal of the American Chemical Society. 128 (19): 6302–6303. doi:10.1021/ja058590u.