Prednicarbate
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604021 |
| Routes of administration | Topical |
| ATC code | D07AC18 (WHO) |
| Identifiers | |
| |
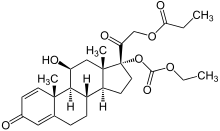
| Synonyms | [2-[(8S,9S,10R,11S,13S,14S,17R)-17-ethoxycarbonyloxy-11-hydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] propanoate |
| CAS Number |
73771-04-7 |
| PubChem (CID) | 6714002 |
| IUPHAR/BPS | 7605 |
| DrugBank |
DB01130 |
| ChemSpider |
5145991 |
| UNII |
V901LV1K7D |
| ChEMBL |
CHEMBL1200386 |
| ECHA InfoCard | 100.070.516 |
| Chemical and physical data | |
| Formula | C27H36O8 |
| Molar mass | 488.57 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Prednicarbate is a relatively new topical corticosteroid drug. It is similar in potency to hydrocortisone. Compared to other topical corticosteroids, like betamethasone, repeated prednicarbate use does not cause skin atrophy as quickly. Corticosteroids have always been an important part of the pharmacological arsenal of dermatology; however, their tendency to produce side-effects has caused the need to search for new preparations.
It is nonhalogenated.[1]
References
- ↑ Gupta AK, Chow M (2004). "A review of prednicarbate (Dermatop)". Skin Therapy Lett. 9 (10): 5–6, 9. PMID 15657633.
This article is issued from Wikipedia - version of the 5/27/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.