Diethyl ether peroxide
 | |
 | |
| Identifiers | |
|---|---|
| 18321-53-4 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChemSpider | 19985446 |
| |
| |
| Properties | |
| C4H10O3 | |
| Molar mass | 106.12 g/mol |
| Density | 1.005 g/cm3 |
| Boiling point | 62 to 64 °C (144 to 147 °F; 335 to 337 K) at 18.7 hPa (reduced pressure) |
| Hazards | |
| Main hazards | Explosive |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
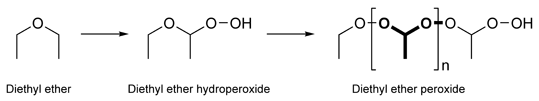
Diethyl ether peroxides are a class of organic peroxides that slowly form in diethyl ether upon storage under air, light, or in the presence of metal by autoxidation.
Diethyl ether hydroperoxide
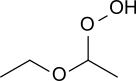
Diethyl ether hydroperoxide (CH3-CH2-O-CH(OOH)-CH3) is a colorless liquid of low viscosity with a pleasant smell. Upon heating it weakly deflagrates, resulting in a fog of acetic acid and water. Diethyl ether hydroperoxide decomposes in the presence of sodium hydroxide and Fe2+-containing salts.
Diethyl ether peroxide, also known as ethylidene peroxide, (-CH(CH3)OO-)n is a polymerization product of diethyl ether hydroperoxide. It is a colorless oily liquid that is an extremely brisant and friction sensitive explosive material. Amounts of less than 5 milligrams can damage chemical apparatuses. The dangerous properties of ether peroxides are the reason that diethyl ether and other peroxide forming ethers like tetrahydrofuran (THF) or ethylene glycol dimethyl ether (1,2-dimethoxyethane) are avoided in industrial processes.
Tests
Diethyl ether peroxides can be detected with a potassium iodide (KI) solution in acetic acid or potassium iodide / starch paper. A positive test results in the formation of iodine (I2) that causes a yellow or brown color of the ether phase or a dark bluish spot on the paper strip.[1]
Degradation
Ether peroxides can be destroyed by disproportionation to acetaldehyde with Fe2+ or Mn2+ ions or with triphenylphosphine (PPh3). The resulting aldehyde has to be removed to prevent a rapid back-formation of peroxides.
References
- A. Rieche, R. Meister, Modellversuche zur Autoxidation der Äther, Angewandte Chemie 49(5):106 (1936) (German)
- ↑ "Peroxide Forming Solvents". Sigma-Aldrich. Retrieved 2014-07-09.
