Echinococcus multilocularis
| Echinococcus multilocularis | |
|---|---|
 | |
| Echinococcus multilocularis isolated from a fox | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Platyhelminthes |
| Class: | Cestoda |
| Order: | Cyclophyllidea |
| Family: | Taeniidae |
| Genus: | Echinococcus |
| Species: | Echinococcus multilocularis |
| Binomial name | |
| Echinococcus multilocularis Leuckart, 1863 | |
Echinococcus multilocularis is a cyclophyllid tapeworm that, along with some other members of the Echinococcus genus (especially E. granulosus), produces the disease known as echinococcosis in certain terrestrial mammals, including wolves, foxes, jackals, coyotes, domestic dogs and humans. Unlike E. granulosus, E. multilocularis produces many small cysts (also referred to as locules) that spread throughout the internal organs of the infected animal. Ingestion of these cysts, usually by a canid eating an infected rodent, results in a heavy infestation of tapeworms.
Signs and symptoms
People infected with E. multilocularis may be asymptomatic for many years. Following the asymptomatic period of this disease, common symptoms are headache, nausea, vomiting, abdominal pain. Jaundice is rare,[1] but hepatomegaly is a common physical finding.
Life cycle
The life cycle of E. multilocularis involves a primary or definitive host and a secondary or intermediate host, each harboring different life stages of the parasite.
Foxes, coyotes, domestic dogs, and other canids are the definitive hosts for the adult stage of the parasite. Cats may also be involved.[2] The head of the tapeworm attaches to the intestinal mucosa by hooks and suckers. It then produces hundreds of microscopic eggs, which are dispersed through the feces.[3]
Wild rodents such as mice serve as the intermediate host. Eggs ingested by rodents develop in the liver, lungs and other organs to form multilocular cysts. Humans could also become an intermediate host by handling infected animals or ingesting contaminated food, vegetable, and water. The life cycle is completed after a fox or canine consumes a rodent infected with cysts. Larvae within the cyst develop into adult tapeworms in the intestinal tract of the definitive host.[3]
Except in rare cases where infected humans are eaten by canines, humans are a dead-end or incidental host (an intermediate host that does not allow transmission to the definitive host) for E. multilocularis.
- Summary of the life cycle
- adult worm present in intestine of definitive host
- eggs passed in feces, ingested by humans or intermediate host
- onchosphere penetrates intestinal wall, carried via blood vessels to lodge in organs
- hydatid cysts develop in liver, lungs, brain, heart
- protoscolices (hydatid sand) ingested by definitive host
- ingested protoscolices attach to small intestine and develops into adult worm
Morphology

The adult parasite is a small tapeworm that is 3- 6mm long, and lives in the small intestine of canines. The segmented worm contains a scolex with suckers and hooks that enable attachment to the mucosal wall, since tapeworms do not have a digestive tract. A short neck connects the head to three proglottids, the body segment of the worm which contains the eggs to be excreted in the feces.[4]
Diagnosis
Serological and imaging tests are commonly used to diagnose this disease. Frequently used serological tests include antibody tests, ELISA and indirect hemaglutination (IHA). Also, an intradermal allergic reaction test (Casoni test) has also been used to diagnose patients. Imaging tests include: X-rays, cat scans, MRI, and ultrasound.[1]
Disease staging
Alveolar echinococcosis (AE) is a highly lethal helminthic disease in humans, caused by the larval form of the parasitic tapeworm E. multilocularis. The disease represents a serious public threat in China, Siberia, and central Europe. However, since the 1990s, the prevalence of the disease seems to be increasing in Europe, not only in the historically endemic areas but its neighboring regions.[5] AE primarily affects the liver by inducing a hepatic disorder similar to liver cancer, therefore becoming extremely dangerous and difficult to diagnose. If the infection metastasizes, it may spread to any other organ and could be lethal if not treated. The most common treatment for AE is to surgically remove the parasite. Since it is difficult and not always possible to remove the entire parasite, medicine such as Albendazole is utilized to keep the cyst from growing back.[3]
Guided by the Tumor-Node-Metastasis (TNM) system of liver cancer, the European Network for Concerted Surveillance of Alveolar Echinococcosis and the World Health Organization Informal Working Group on Echinococcosis, a clinical classification system has been proposed. This classification system has been designated as the "PNM" system (P = parasitic mass, N = involvement of neighboring organs, M = metastasis). The system was developed by a retrospective analysis of records from 97 patients treated in France and Germany (2 treatment centers). Amongst other characteristics, the system takes into consideration the localization of the parasite in the liver, the extent of lesion involvement, regional involvement, and metastasis.[6]
Treatment
If no specific therapy is initiated, in 94% of patients the disease is fatal within 10–20 years following diagnosis.[7]
- Currently, benzimidazoles (such as albendazole) are used to treat AE: only halt their proliferation and do not actually kill the parasites, side effects such as liver damage
- 2-ME2, a natural metabolite of estradiol, is tested with some results in vitro: decreased transcription of 14-3-3-pro-tumorogenic zeta-isoform, causes damage to germinal layer but does not kill parasite in vivo
- Treatment with a combination of albendazole/2-ME2 showed best results in reducing parasite burden[8]
- Despite the improvements in the chemotherapy of echinococcosis with benzimidazole derivatives, complete elimination of the parasitic mass cannot be achieved in most infected patients, although studies indicate that long-term treatment with mebendazole may cause the death of the parasite. ---> Which reference does this come from?
Epidemiology
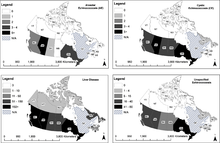
The incidence of human infestation with E. multilocularis and disease is increasing in urban areas, as wild foxes (an important reservoir species of the sylvatic cycle) are migrating to urban and suburban areas and gaining closer contact with human populations.[3] Also, restocking fox enclosures for fox hunting with infected animals spreads the disease.[10] Children, health care workers and domestic animals are at risk of ingesting the cysts after coming into contact with the feces of infected wild foxes. Even with the improvement of health in developed/industrialized countries, the prevalence of alveolar echinococcosis (AE) did not decrease.[3] On the contrary, incidents of AE have now also been registered in eastern European countries and sporadic incidences in other European countries.[3]
The disease has extended its range in Europe in the last few decades.[11] Still the infection is fairly rare. Between 1982 and 2000 a total of 559 cases were reported throughout Europe.[12]
Recent findings indicate that E. multilocularis is likely expanding its range in the central region of the United States and Canada and that invasions of European strains might have occurred; the endemic presence of the parasite in urban areas and a recent human case in Alberta, Canada have been reported.[9]
See also
References
- 1 2 Kayacan SM, Vatansever S, Temiz S, et al. (January 2008). "Alveolar echinococcosis localized in the liver, lung and brain". Chin. Med. J. 121 (1): 90–2. PMID 18208675.
- ↑ Knapp, Jenny; Combes, Benoît; Umhang, Gérald; Aknouche, Soufiane; Millon, Laurence (2016). "Could the domestic cat play a significant role in the transmission of Echinococcus multilocularis? A study based on qPCR analysis of cat feces in a rural area in France". Parasite. 23: 42. doi:10.1051/parasite/2016052. ISSN 1776-1042. PMID 27739398.

- 1 2 3 4 5 6 Echinococcosis at eMedicine
- ↑ John, David T.; William Petri, William A.; Markell, Edward K.; Voge, Marietta (January 2006). "7: The Cestodes: Echinococcus granulosus, E. multiloularis and E. vogeli (Hyatid Disease)". Markell and Voge's Medical Parasitology (9th ed.). Elsevier Health Sciences. pp. 224–231. ISBN 0-7216-4793-6.
- ↑ Torgerson PR, Keller K, Magnotta M, Ragland N (2010). "The global burden of alveolar echinococcosis". PLoS Negl Trop Dis. 4 (6): e722. doi:10.1371/journal.pntd.0000722. PMC 2889826
 . PMID 20582310.
. PMID 20582310. - ↑ Kern P, Wen H, Sato N, et al. (2006). "WHO classification of alveolar echinococcosis: principles and application". Parasitol. Int. 55 (Suppl): S283–7. doi:10.1016/j.parint.2005.11.041. PMID 16343985.
- ↑ Jura H, Bader A, Frosch M (May 1998). "In vitro activities of benzimidazoles against Echinococcus multilocularis metacestodes". Antimicrob. Agents Chemother. 42 (5): 1052–6. PMC 105743
 . PMID 9593125.
. PMID 9593125. - ↑ Spicher M, Naguleswaran A, Ortega-Mora LM, Müller J, Gottstein B, Hemphill A (March 2008). "In vitro and in vivo effects of 2-methoxyestradiol, either alone or combined with albendazole, against Echinococcus metacestodes". Exp. Parasitol. 119 (4): 475–82. doi:10.1016/j.exppara.2008.02.012. PMID 18442817.
- 1 2 Massolo, Alessandro; Liccioli, Stefano; Budke, Christine; Klein, Claudia (2014). "Echinococcus multilocularis in North America: the great unknown". Parasite. 21: 73. doi:10.1051/parasite/2014069. ISSN 1776-1042. PMC 4273702
 . PMID 25531581.
. PMID 25531581. 
- ↑ Drisdelle, Rosemary (2010). Parasites: Tales of Humanity's Most Unwelcome Guests. University of California Press. p. 96. ISBN 978-0-520-25938-6.
- ↑ Sréter T, Széll Z, Sréter-Lancz Z, Varga I (July 2004). "Echinococcus multilocularis in Northern Hungary". Emerging Infect. Dis. 10 (7): 1344–6. doi:10.3201/eid1007.031027. PMC 3323332
 . PMID 15338552.
. PMID 15338552. - ↑ Kern P, Bardonnet K, Renner E, et al. (March 2003). "European echinococcosis registry: human alveolar echinococcosis, Europe, 1982–2000". Emerging Infect. Dis. 9 (3): 343–9. doi:10.3201/eid0903.020341. PMC 2958541
 . PMID 12643830.
. PMID 12643830.
External links
- Echinococcus multilocularis
- "Echinococcus multilocularis". NCBI Taxonomy Browser. 6211.