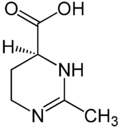
Ectoine
 | |
| Names | |
|---|---|
| IUPAC name
(S)-2-methyl-3,4,5,6-tetrahydropyrimidine-4-carboxylic acid | |
| Other names
THP(B) | |
| Identifiers | |
| 96702-03-3 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:27592 |
| ChemSpider | 112069 |
| KEGG | C06231 |
| PubChem | 126041 |
| UNII | 7GXZ3858RY |
| |
| |
| Properties | |
| C6H10N2O2 | |
| Molar mass | 142.16 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Ectoine (1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid) is a natural compound found in several species of bacteria. It is a compatible solute which serves as a protective substance by acting as an osmolyte and thus helps organisms survive extreme osmotic stress. Ectoine is found in high concentrations in halophilic microorganisms and confers resistance towards salt and temperature stress. Ectoine was first identified in the microorganism Ectothiorhodospira halochloris,[2][3] but has since been found in a wide range of Gram-negative and Gram-positive bacteria. Other species of ectoine were found in
- Brevibacterium linens[3]
- Halomonas elongata[2][4]
- Marinococcus halophilus[5]
- Pseudomonas stutzeri[4]
Biosynthesis
Ectoine is synthesized in three successive enzymatic reactions starting from aspartic β-semialdehyde. The genes involved in the biosynthesis are called ectA, ectB and ectC and they encode the enzymes L-2,4-diaminobutyric acid acetyltransferase, L-2,4-diaminobutyric acid transaminase, and L-ectoine synthase, respectively.[5][4]
Use
Ectoine is used as an active ingredient in skin care and sun protection products. It stabilizes proteins and other cellular structures and protects the skin from stresses like UV irradiation and dryness.[4]
References
- ↑ Ectoine at Sigma-Aldrich
- 1 2 Peters, P; Miwatani, T; Honda, T (1990). "The biosynthesis of ectoine". FEMS Microbiology Letters. 71 (2): 157–61. doi:10.1016/0378-1097(90)90049-V. PMID 1601286.
- 1 2 Bernard, T.; Jebbar, M.; Rassouli, Y.; Himdi-Kabbab, S.; Hamelin, J.; Blanco, C. (1993). "Ectoine accumulation and osmotic regulation in Brevibacterium linens" (PDF). Journal of General Microbiology. 139: 129. doi:10.1099/00221287-139-1-129.
- 1 2 3 4 Stöveken, N; Pittelkow, M; Sinner, T; Jensen, R. A.; Heider, J; Bremer, E (2011). "A specialized aspartokinase enhances the biosynthesis of the osmoprotectants ectoine and hydroxyectoine in Pseudomonas stutzeri A1501". Journal of Bacteriology. 193 (17): 4456–68. doi:10.1128/JB.00345-11. PMC 3165526
 . PMID 21725014.
. PMID 21725014. - 1 2 Louis, P; Galinski, E. A. (1997). "Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli". Microbiology. 143 ( Pt 4) (4): 1141–9. doi:10.1099/00221287-143-4-1141. PMID 9141677.