Frenkel defect
A Frenkel defect is a type of defect in crystalline solids wherein an atom is displaced from its lattice position to an interstitial site, creating a vacancy at the original site and an interstitial defect at the new location within the same element without any changes in chemical properties.[1]
Definition
A Frenkel defect, Frenkel pair, or Frenkel disorder is a type of point defect in a crystal lattice. The defect forms when an atom or smaller ion (usually cation) leaves its place in the lattice, creating a vacancy, and becomes an interstitial by lodging in a nearby location.[2] Their prime mechanism of generation is by particle irradiation, as their equilibrium concentration according to the Boltzmann distribution is much smaller than the pure vacancies distribution, due to the large energy necessary for the creation of the associated interstitial atoms. The phenomenon is named after the Soviet physicist Yakov Frenkel, who discovered it in 1926.[3]
Effect on density
This defect does not have any impact on the density of the solid as it involves only the migration of the ions within the crystal, thus preserving both the volume as well as mass.
Examples
Frenkel defects are exhibited in ionic solids with a large size difference between the anion and cation (with the cation usually smaller due to an increased effective nuclear charge)
Some examples of solids which exhibit Frenkel defects:
- zinc sulfide,
- silver(I) chloride,
- silver(I) bromide (also shows Schottky defects),
- silver(I) iodide.
These are due to the comparatively smaller size of Zn2+ and Ag+ ions.
For example, consider a lattice formed by Xn+ and Mn+ ions. Suppose an M ion leaves the M sublattice, leaving the X sublattice unchanged. The number of interstitials formed will equal the number of vacancies formed.
One form of a Frenkel defect reaction in MgO with the oxide anion leaving the lattice and going into the interstitial site written in Kröger–Vink notation:
- Mg×
Mg + O×
O → O′′
i + v••
O + Mg×
Mg
This can be illustrated with the example of the sodium chloride crystal structure. The diagrams below are schematic two-dimensional representations.
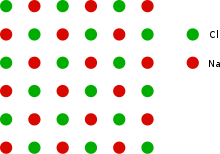
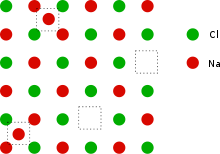
See also
References
- ↑ Chiang, Yet-Ming; Birnie III, Dunbar; Kingery, W. David (1997). Physical Ceramics: Principles for Ceramic Science and Engineering (1st ed.). John Wiley & Sons. pp. 102–107. ISBN 0-471-59873-9.
- ↑ Ashcroft and Mermin (1976). Solid State Physics. Cengage Learning. p. 620. ISBN 0030839939.
- ↑ Frenkel, Yakov (1926). "Über die Wärmebewegung in festen und flüssigen Körpern (About the thermal motion in solids and liquids)". Zeitschrift für Physik. Springer. 35 (8): 652–669. Bibcode:1926ZPhy...35..652F. doi:10.1007/BF01379812. Retrieved 21 October 2015.
Further reading
- Kittel, Charles (2005). Introduction to Solid State Physics (8th ed.). Wiley. pp. 585–588. ISBN 0-471-41526-X.