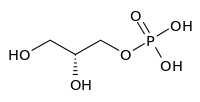
Glycerol 3-phosphate
 | |
| Names | |
|---|---|
| IUPAC name
(R)-2,3-dihydroxypropyl dihydrogen phosphate | |
| Other names
1,2,3-propanetriol, 1-(dihydrogen phosphate), (2R)- D-glycerol 1-phosphate L-glycerol 3-phosphate L-α-glycerophosphate L-α-phosphoglycerol | |
| Identifiers | |
| 17989-41-2 | |
| 3D model (Jmol) | Interactive image |
| ECHA InfoCard | 100.000.279 |
| KEGG | C00093 |
| MeSH | Alpha-glycerophosphoric+acid |
| PubChem | 439162 |
| |
| |
| Properties | |
| C3H9O6P | |
| Molar mass | 172.074 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
sn-Glycerol 3-phosphate[1] is a phosphoric ester of glycerol, which is a component of glycerophospholipids. Equally appropriate names in biochemical context include glycero-3-phosphate, 3-O-phosphonoglycerol, 3-phosphoglycerol.[2] From a historical reason, it is also known as L-glycerol 3-phosphate, D-glycerol 1-phosphate, L-α-glycerophosphoric acid.[2] It should not be confused with the similarly named glycerate 3-phosphate or glyceraldehyde 3-phosphate.
Biosynthesis and metabolism
Glycerol 3-phosphate is synthesized by reducing dihydroxyacetone phosphate (DHAP), a glycolysis intermediate, with glycerol-3-phosphate dehydrogenase. DHAP and thus glycerol 3-phosphate is also possible to be synthesized from amino acids and citric acid cycle intermediates via glyceroneogenesis pathway.
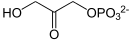 + NAD(P)H + H+ →
+ NAD(P)H + H+ →  + NAD(P)+
+ NAD(P)+
It is also synthesized by phosphorylating glycerol generated upon hydrolyzing fats with glycerol kinase, and can feed into glycolysis or glyconeogenesis pathways.
Glycerol 3-phosphate is a starting material for de novo synthesis of glycerolipids. In eukaryotes, it is first acylated on its sn-1 position by an ER- or mitochondrial membrane enzyme, glycerol-3-phosphate O-acyltransferase, and another acyl group is then added on the sn-2 position making phosphatidic acids.
 + Acyl-CoA → Lysophosphatidic acid + CoA
+ Acyl-CoA → Lysophosphatidic acid + CoA
Some fungi have glycerol-1-phosphatase, which removes the phosphate group of glycerol 3-phosphate to generate glycerol. They can perform glycerol fermentation producing glycerol from glucose through glycolysis pathway.
 + H2O →
+ H2O →  + Pi
+ Pi
Shuttle system
Glycerol-3-phosphate dehydrogenases are located both in the cytosol and the intermembrane face of mitochondrial inner membrane. Glycerol 3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP) are so small molecule that they can permeate mitochondrial outer membrane through porins and shuttle between two dehydroganases. Using this shuttle system, NADH generated by cytosolic metabolisms including glycolysis are reoxidized to NAD+ reducing DHAP to G3P, and the reducing equivalent can be used for generating proton gradient across the mitochondrial inner membrane by coupling oxidizing G3P and reducing quinone.
Enantiomer
Glycerol 1-phosphate, sometimes called as D-glycerol 3-phosphate, is an enantiomer of glycerol 3-phosphate. Most organisms use 3-phosphate, or L-configuration, for glycerolipid backborn; however, 1-phosphate is specifically used in archeal ether lipids.
Notes
- ↑ This article uses stereospecific numbering where stereoconfiguration is not explicitly specified.
- 1 2 G. P. Moss (ed.). "Nomenclature of Phosphorus-Containing Compounds of Biochemical Importance". Retrieved 2015-05-20.