Illite
| Illite | |
|---|---|
 | |
| General | |
| Category | Mica - Phyllosilicates |
| Formula (repeating unit) | (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2O)] |
| Strunz classification | 9.EC.60 |
| Dana classification | 71.02.02d.02 |
| Crystal system | Monoclinic |
| Crystal class |
Prismatic (2/m) H-M symbol: (2/m) |
| Identification | |
| Color | Grey-white to silvery-white, greenish-gray |
| Crystal habit | Micaceous aggregates |
| Cleavage | {001} Perfect |
| Mohs scale hardness | 1 - 2 |
| Luster | Pearly to dull |
| Streak | white |
| Diaphaneity | Translucent |
| Specific gravity | 2.6 - 2.9 |
| Optical properties | Biaxial (-) |
| Refractive index | nα = 1.535 - 1.570 nβ = 1.555 - 1.600 nγ = 1.565 - 1.605 |
| References | [1][2] |
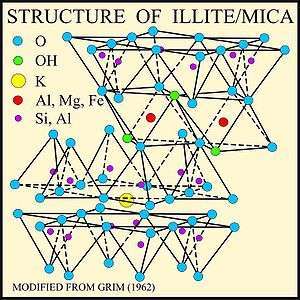
Illite is a non-expanding clay crystalline mineral. Illite is a secondary mineral precipitate phyllosilicate or layered alumino-silicate. Its structure is a 2:1 clay of silica tetrahedron – alumina octahedron – silica tetrahedron layers.[4] The space between individual clay crystals is occupied by poorly hydrated potassium cations which is responsible for the absence of swelling. Structurally, illite is quite similar to muscovite with slightly more silicon, magnesium, iron, and water and slightly less tetrahedral aluminium and interlayer potassium. The chemical formula is given as (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2O)],[2] but there is considerable ion (isomorphic) substitution. It occurs as aggregates of small monoclinic grey to white crystals. Due to the small size, positive identification usually requires x-ray diffraction or SEM-EDS (automated mineralogy) analysis. Illite occurs as an altered product of muscovite and feldspar in weathering and hydrothermal environments; it may be a component of sericite. It is common in sediments, soils, and argillaceous sedimentary rocks as well as in some low grade metamorphic rocks. The iron rich member of the illite group, glauconite, in sediments can be differentiated by x-ray analysis.[4]
The cation exchange capacity (CEC) of illite is smaller than that of smectite but higher than that of kaolinite, typically around 20 – 30 meq/100 g.
Illite was first described for occurrences in the Maquoketa shale in Calhoun County, Illinois, US, in 1937. The name was derived from its type location in Illinois.[1]
Illite is also called hydromica or hydromuscovite. Brammallite is a sodium rich analogue. Avalite is a chromium bearing variety which has been described form Mt. Avala, Belgrade, Serbia.[5]
Illite crystallinity
The crystallinity of illite has been used as an indicator of metamorphic grade in clay-bearing rocks metamorphosed under conditions between diagenesis and low-grade metamorphism.[6] With increasing temperature, illite is thought to undergo a transformation into muscovite.[7]
References
- Mitchell J.K. (1993) Fundamentals of soil behavior. Second edition. John Wiley and Sons, Inc., New York. 437 pp, see Chapter 3, Soil Mineralogy, p. 32. ISBN 978-0-471-46302-3
- 1 2 http://www.mindat.org/min-2011.html Mindat
- 1 2 http://webmineral.com/data/Illite.shtml
- ↑ Crystal Structures of Clay Minerals and Their X-ray Identification, George William Brindley, George Brown, 1980 https://books.google.co.uk/books/about/Crystal_Structures_of_Clay_Minerals_and.html?id=KhAJAQAAIAAJ&redir_esc=y
- 1 2 http://pubs.usgs.gov/of/2001/of01-041/htmldocs/clays/illite.htm Illite group, USGS
- ↑ http://www.mindat.org/min-435.html Mindat - avalite
- ↑ M. Frey; Douglas Robinson (26 January 1999). Low-Grade Metamorphism. Wiley. pp. 61–107. ISBN 978-0-632-04756-7.
- ↑ Gharrabi, M., Velde, B. & Sagon, J.-P. 1998. The transformation of illite to muscovite in pelitic rocks : Constraints from X-ray diffraction, Clays and clay minerals, 46, 79-88.
| Wikimedia Commons has media related to Illite. |