Inositol-trisphosphate 3-kinase
| Inositol trisphosphate 3-kinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Inositol-trisphosphate 3-kinase A Catalytic Core. 1TZD | |||||||||
| Identifiers | |||||||||
| EC number | 2.7.1.127 | ||||||||
| CAS number | 106283-10-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
Inositol (1,4,5) Trisphosphate-3- Kinase (EC 2.7.1.127) is an enzyme that facilitates a phospho-group transfer from ATP to 1D-myo-inositol 1,4,5-trisphosphate. The first evidence of this kinase was isolated in 1990 from rat brain, completing the inositol metabolism pathway. Since this isolation, 3 human isoforms have been found (A,B,C). This enzyme (all isoforms) plays a vital role in the calcium signalling pathway by terminating the propagation of the signal caused by IP3 by converting it to IP4. ITP3K is regulated directly by Calmodulin directly and Calcium-Calmodulin dependent kinase II, as well as PKA and PKC. ITP3K itself is being targeted in anti-cancer drug efforts. Also, because of its role in metabolism, ITP3K is involved in the therapeutic use of inositol when taken for bipolar disorder, panic disorder, prevention of Spina Bifida of fetuses, reduce the onset of diabetes in pregnant women, and decrease tumor size in lung cancer.
Nomenclature
This enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups (phosphotransferases) with an alcohol group as acceptor. The systematic name of this enzyme class is ATP:1D-myo-inositol-1,4,5-trisphosphate 3-phosphotransferase. Other names in common use include 1D-myo-inositol-trisphosphate 3-kinase, and Ins(1,4,5)P3 3-kinase.
Discovery
It was first reported that inositol was metabolized and absorbed by the body in 1934;[1] however, it was not until 1986 that the full metabolic pathway was theorized. It was not certain which kinase was responsible for the conversion of inositol (1,4,5) trisphosphate (abbreviated IP3) to inositiol (1,3,4,5)tetrakisphosphate (abbreviated IP4) until the first isolation of ITP3K in 1990.[2] It was later found in 1993 that IP3 is an important second messenger in cell signaling events like "including fertilization, cell growth, transformation, secretion, smooth muscle contraction, sensory perception and neuronal signalling.[2]"
Structure
.png)

In mammalian Inositol-trisphosphate 3-kinases, there are two major functional domains. These are a highly conserved C-terminal catalytic domain and a divergent N-terminal regulatory domain. The catalytic core further consists of two domains as well which play a role in binding with residues and ATP.[2]
Catalytic domain
The structure of the catalytic domain of the Human Inositol-Trisphosphate 3-Kinase has been shown to be divided into three subdomains. These subdomains are displayed as the N lobe, which is a N-terminal domain, the C lobe, which is a C-terminal subdomain and a third alpha-only subdomain.[3]
The catalytic domain of Inositol-Trisphosphate 3-Kinase contains a catalytic domain which varies from the protein kinase superfamily, as well as a novel four-helix substrate binding domain. In this kinase, the two domains are in an open conformation, which indicates that the two domains are both accessible at the same time. This suggests that substrate recognition and catalysis by Inositol-Trisphosphate 3-Kinase involves a dynamic conformational cycle. Additionally, this unique helical domain of Inositol Trisphosphate 3 Kinase blocks access to the active site by membrane-bound phosphoinositides, explaining the structural basis for soluble inositol polyphosphate specificity. Another feature of the catalytic core is the ATP binding site. Here, one molecule of ADP is bound in the cleft of the major domain, which indicates the active site of the kinase.[3]
In further detail, the larger domain of the protein structure has an α/β-class structure. The domain has an N-terminal and a C-terminal lobe with a cleft in between and each of these lobes is built around an antiparallel β-sheet. In the N-terminal, the sheet has three strands, whereas in the C-terminal there is a five-stranded sheet. The second domain, is α-helical and consists of four α helices linked by long loops. The helices are loosely packed against each other and the entire domain is highly mobile as compared to the large α/β domain. The helical domain is juxtaposed against one end of the cleft in the large domain [3]
Isoforms
There are three Inositol Trisphosphate 3 Kinases which are encoded by the human genome. These are Inositol Trisphosphate 3 Kinases A, B, and C. All of the kinase isoforms have similar C-terminal catalytic domain and share common structural features. Isoform Inositol Trisphosphate 3 Kinase A is predominant in neurons and in the testes. It is localized to dendritic spines by an association with filamentous actin which is consistent with its probable role in memory functions. Isoform Inositol Trisphosphate 3 Kinase B is expressed more widely, and it is found in the cytosol in addition to the endoplasmic reticulum and actin. Lastly, Inositol Trisphosphate 3 Kinase C is also expressed in many different tissues and is partially nuclear. In addition, it is activated by CaM to a lesser degree than the A or B isoforms.[2]
There have been at least three distinct isoforms of Inositol-trisphosphate 3-kinase which have been discovered. These isoforms differ in their molecular masses, Ca2+/calmodulin (Ca2+/CaM) sensitivity, intracellular distribution and tissue expression. Generally, mammalian Inositol-trisphosphate 3-kinases are activated by calcium and calmodulin to varying degrees. The method in which this works is calmodulin recognizes sequences which contain amphiphilic alpha-helices with clusters of positively charged and hydrophobic amino acids. Certain sequences are required for CaM binding and enzyme activation and this level of stimulation appears to be specific to cell, tissue, and isoform. Enzymatic activities by calcium and calmodulin can be observed in mammalian ITP3Ks, however, ITP3K's from nematodes and Arabidopsis thaliana lack the CaM-binding sites and therefore are insensitive to calcium and calmodulin.[2]
In terms of why isoforms exist, they are part of nature and they serve a specific function. There are several existing isoforms of Inositol Trisphosphate 3 Kinase, each serving a specific role in metabolic control, immunity, angiogenesis, and cardiovascular homeostasis. These isoforms exist because the different chemical structure of each isoform allows for different bond-to-bond interactions, which then allows phosphorylation to occur in different times/manners.
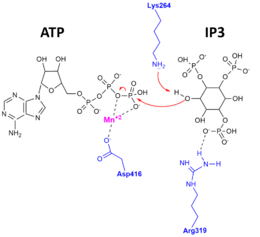
Function
Inositol 1,4,5-trisphosphate 3-kinase (ITP3K) catalyzes the transfer of the gamma-phosphate from ATP to the 3-position of inositol 1,4,5-trisphosphate to form inositol 1,3,4,5-tetrakisphosphate.[4] ITP3K is highly specific for the 1,4,5-isomer of IP3, and it exclusively phosphorylates the 3-OH position. Evidence for this exquisite specificity and for the catalytic mechanism was found when the apo-enzyme, substrate-bound complex, and product-bound complex X-ray crystal structures of ITP3K were determined.[5] The figure to the right depicts the catalytic mechanism, whereby the 3'OH of IP3 attacks the gamma-phosphate of ATP, and amino acid residues of ITP3K important for stabilizing the substrates and products in the active site.
Role in calcium signaling pathway
ITP3K is plays a role in the calcium signaling pathway. In this pathway, either a G-protein coupled receptor (GPCR) or receptor tyrosine kinase (RTK) is activated by an extracellular ligand-binding event. Initiation of the pathway leads to an activated G-alpha subunit of a heterotrimeric G protein (in the case of GPCR-mediated signal transduction) or autophoshorylation of RTK cytoplasmic domains (in the case of RTK-mediated signal transduction). These intracellular events eventually lead to activation of phospholipase C (PLC), which cleaves the phospholipid PIP2 into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). DAG remains associated with the plasma membrane, while IP3 is released into the cytoplasm. IP3 then goes on to bind to IP3 receptors on the endoplasmic reticulum or sarcoplasmic reticulum, resulting in an influx of calcium ions into the cytoplasm.[6]
Calcium serves as a second messenger for various downstream cellular events including glycogen metabolism, muscle contraction, neurotransmitter release, and transcriptional regulation.[6] Therefore, calcium homeostasis is essential for proper cell function and response to extracellular signals.[7]
In order to prepare the cell for a future signaling event, the calcium pathway must be tightly regulated. ITP3K seems to play an important role in termination of the signal. As mentioned, ITP3K catalyzes the phosphorylation of IP3 to make IP4. Unlike IP3, IP4 does not cause opening of calcium channels on the endoplasmic reticulum or sarcoplasmic reticulum.[8] By decreasing the concentration of IP3 in the cytoplasm, ITP3K terminates propagation of the calcium signaling pathway.[2]

Additional roles
ITP3K is not the only enzyme responsible for clearing IP3 from the cytoplasm. A second enzyme called inositol 5-phosphatase catalyzes the dephosphorylation of IP3 to create IP2.[9] Typically, nature does not favor the evolution of a second enzyme to perform an already-existing, identical function.[10] A closer inspection of the evolutionary history of inositol 5-phosphatase and ITP3K gives rise to several interesting hypotheses about the roles of these enzymes in the cell.
Inositol 5-phosphatase existed before ITP3K evolved in the mammalian cell. Like other phosphatases, inositol 5-phosphatase is an energy-independent enzyme that cleaves a phosphate group off of a substrate.[11] In contrast, ITP3K (like all kinases) is energy-dependent, meaning that it requires an ATP molecule to perform the phosphoryl transfer chemistry.[12] If nature already had an energy-independent mechanism for termination of the calcium signaling pathway, why was the evolution of ITP3K advantageous? This apparent redundancy of function, or "waste" of energy by the cell, suggests that ITP3K may have a more important function in the cell than simply clearing the IP3 second messenger from the cytoplasm.[11] Current hypotheses about additional roles for ITP3K are explained in the following two subsections.
Product of ITP3K may be a second messenger
As mentioned previously, ITP3K catalyzes a phosphoryl transfer reaction that converts IP3 to IP4. IP4 does not stimulate calcium influx through IP3 receptor channels on the endoplasmic or sarcoplasmic reticulum. However, it has been shown that IP4 stimulates calcium channel opening on the plasma membrane.[8] In this way, IP4 may actually serve to prolong the calcium signal by activating the influx of calcium stores from the extracellular space.
In addition, there is evidence that IP4 binds two GTPase-activating proteins, GAP1IP4BP and GAP1m.[9] GAPs are often used in signal transduction as on/off switches. IP4 binding to GAPs suggests that ITP3K may be involved in a parallel signal transduction pathway.[8] The exact role of IP4 binding to these GAPs has not been determined, though, so additional research in this area will be needed to gain a more complete understanding.
Role in inositol phosphate metabolism
In addition to its potential roles as a second messenger, IP4 may also function as an essential precursor for other more highly phosphorylated inositol phosphates such as IP5, IP6, IP7, and IP8.[2] These higher order inositol phosphates are thought to be important for phosphate storage, may serve as additional second messengers, and may be involved in the post-signal recovery phase whereby calcium stores are refilled and the phosphatidylinositol supply is replenished.[8] Such maintenance is necessary to prepare the cell for a future incoming signal.
The generation of the higher order inositol phosphates discussed above relies on the presence of their precursor, IP4. ITP3K is responsible for the formation of IP4. Therefore, it is possible that one of ITP3K’s most important functions is the regulation of IP4 levels, and therefore the regulation of downstream cellular events involving higher order inositol phosphates.
Regulation of ITP3K
ITP3K is regulated by various post-translational mechanisms. The major post-translational modification that is important for ITP3K regulation is phosphorylation. Phosphorylation is one of the most widespread protein modifications, and it is especially common in the modulation of signal transduction pathways. ITP3Ks are stimulated directly by calcium/calmodulin (Ca2+/CaM) binding.[2] In addition, ITP3K activity is indirectly stimulated by phosphorylation by calcium/calmodulin-dependent kinase II (CaMKII).[11] In addition, there is evidence that ITP3Ks may be activated upon phosphorylation by protein kinase C (PKC) and inhibited upon phosphorylation by protein kinase A (PKA),[8] but the degrees of these interactions remain elusive.
Therapeutic use
Inositol can be used for treatment in a variety of conditions and as a preventative. In the metabolism of this compound, ITP3K is important for its conversion to IP4; however, it has been suggested that ITP3K itself could also be a "druggable" protein target. A druggable target means that this kinase would be inhibited or activated by a small molecule to manipulate the pathways it is involved in.
Generating stem cells
The kinase itself has been identified as a possible route for generating pluripotent stem cells.[13] Researchers have been trying to inhibit kinases, which were activated by the gene manipulation used to generate pluripotent stem cells. The inhibition of ITP3K, AurkA, and P38 expedited the method used to make these induced pluripotent stem cells (iPSC). The potential in this field could possibly find treatments for many types of cancers and neurodegenerative disorders.
In 2015, it was reported that 226 companies are working on B cell receptor signalling pathways.[14] The pipeline includes 186 drug targets; the goal is activation of non-receptor tyrosine kinases such as Src, Tek, and Syc family of kinases. The downstream messengers of these pathways include DAG, MAP/ERK, JNK, and IP3.
Psychiatry
In the late 1990s, it was thought that inositol could be given as a pseudo-vitamin because of its importance in multiple biological processes. Particularly, inositol is converted to a second messenger for several muscarnic cholinergic receptors. A trial indicated that inositol treatment could be used in the spectrum of illnesses that are benefited by serotonin selective re-uptake inhibitors, such as depression, panic, and OCD. However, it was found that this treatment was not beneficial in Alzheimer's, ADDH, schizophrenia, and autism.[15] It is important to note that no full scale clinical trial has been performed to confirm inositol as a treatment for any psychiatric disorder; though, it remains a suggestion as an alternative medicine by the Mayo Clinic for panic disorders.[16]
In 2014, a pilot study suggested that a combination of Omega-3-fatty acids and inositol aided in the treatment of bipolar spectrum disorders in children ages 6–12.[17] Importantly, this combination was similar to the drugs prescribed without the side effects. The exact method of action for inositol in this context is not known; they speculate that inositol works through a cascade of post synaptic events. This is consistent with the calcium signalling pathway that is modulated by metabolism of inositol, which includes ITP3K.
Pregnancy
In 2010, scientists suggested inositol could be taken as a supplement to prevent the birth defect Spina Bifida[18] Normally, expecting mothers are advised to take folic acid in prevention of such neural tube disorders; researchers from the Institute of Child Health (University College, London) advice taking folic acid in addition to inositol. In vivo mice studies suggest inositol stimulates tissue growth and that is the mechanism of prevention.
Italian researchers have found that D-inositol given to pregnant women with polycistic ovarian syndrome (PCOS) can improve insulin sensitivity and[19] glycaemic control. PCOS affects 10% of women and is characterized by excess levels of male hormone, which can lead to insulin resistance. This study showed that D-inositol reduced the onset of gestational diabetes and reduced the total weight gained during pregnancy. Inositol is metabolized into the important cellular signals IP4, IP5, and IP6 by ITP3K and other proteins.
Lung cancer
Side effects of cigarette smoke are damaged- gene products that regulate cellular function; the inflicted damage prevents normal cell regulation. In tumor cell growth, NF-kappaB mediates metastasis, proliferation, and invasion. An in vivo mouse study showed that there was a minimal decrease in lung tumor size after mice were exposed to cigarette smoke for 5 months and fed a diet of myo-inostol. The metabolism of inositol (and myo-inositol) is partly done by ITP3K.
References
- ↑ MacLellan B (1976). "Matthew my son: prepared childbirth at the General". Can Nurse. 72 (3): 38–9. PMID 1253147.
- 1 2 3 4 5 6 7 8 Xia HJ, Yang G (Feb 2005). "Inositol 1,4,5-trisphosphate 3-kinases: functions and regulations". Cell Research. 15 (2): 83–91. doi:10.1038/sj.cr.7290270. PMID 15740635.
- 1 2 3 Miller GJ, Hurley JH (Sep 2004). "Crystal structure of the catalytic core of inositol 1,4,5-trisphosphate 3-kinase". Molecular Cell. 15 (5): 703–711. doi:10.1016/j.molcel.2004.08.005. PMID 15350215.
- ↑ "UniProtKB - P23677 (IP3KA_HUMAN)". Retrieved 19 February 2015.
- ↑ González B, Schell MJ, Letcher AJ, Veprintsev DB, Irvine RF, Williams RL (Sep 2004). "Structure of a human inositol 1,4,5-trisphosphate 3-kinase: substrate binding reveals why it is not a phosphoinositide 3-kinase". Molecular Cell. 15 (5): 689–701. doi:10.1016/j.molcel.2004.08.004. PMID 15350214.
- 1 2 Berridge MJ (Jan 1993). "Inositol trisphosphate and calcium signalling". Nature. 361 (6410): 315–325. doi:10.1038/361315a0. PMID 8381210.
- ↑ Voet, Donald Voet, Judith G. (2011). Biochemistry (4th ed.). Hoboken, NJ: John Wiley & Sons. ISBN 978-0-470-57095-1.
- 1 2 3 4 5 Havas N (Aug 2011). "Back in the water". Journal of Palliative Medicine. 14 (8): 327–338. doi:10.1089/jpm.2011.0043. PMID 21809925.
- 1 2 Pattni K, Banting G (Jun 2004). "Ins(1,4,5)P3 metabolism and the family of IP3-3Kinases". Cellular Signalling. 16 (6): 643–654. doi:10.1016/j.cellsig.2003.10.009. PMID 15093605.
- ↑ "Understanding Evolution". Retrieved 19 February 2015.
- 1 2 3 Irvine RF, Lloyd-Burton SM, Yu JC, Letcher AJ, Schell MJ (2006). "The regulation and function of inositol 1,4,5-trisphosphate 3-kinases". Advances in Enzyme Regulation. 46 (1): 314–323. doi:10.1016/j.advenzreg.2006.01.009. PMID 16857241.
- ↑ "WikiKinome". Kinase.com. Retrieved 19 February 2015.
- ↑ "Making it easier to make stem cells". Medical XPress. Sanford-Burnham Medical Research Institute. September 5, 2012. Retrieved January 31, 2015.
- ↑ Wood, Laura (January 28, 2015). "Research and Markets: B Cell Receptor Signaling Pathway in Oncology Drug Pipeline Update 2015: 226 companies plus partners developing 295 targeting drugs in 1308 developmental projects". Business Wire. Retrieved February 1, 2015.
- ↑ Levine J (May 1997). "Controlled trials of inositol in psychiatry". European Neuropsychopharmacology : The Journal of the European College of Neuropsychopharmacology. 7 (2): 147–155. doi:10.1016/S0924-977X(97)00409-4. PMID 9169302.
- ↑ "Panic Attacks and Panic Disorders- Alternative Medicine". Mayo Clinic. Mayo Foundation for Education and Research. Retrieved 2015-01-29.
- ↑ Lowry, Fran (June 26, 2014). "Omega-3 Fatty Acids Plus Inositol Promising in Pediatric PBD". MedScape. Retrieved January 31, 2015.
- ↑ Bradford, Eleanor (9 September 2010). "Vitamin 'may help prevent' spina bifida". BBC News. Retrieved 2015-01-31.
- ↑ Costantino D, Guaraldi C (Jun 2014). "[Role of D-chiro-inositol in glucidic metabolism alterations during pregnancy]". Minerva Ginecologica. 66 (3): 281–91. PMID 24971783.
Further reading
- Hansen CA, Mah S, Williamson JR (Jun 1986). "Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver". The Journal of Biological Chemistry. 261 (18): 8100–3. PMID 3487541.
- Irvine RF, Letcher AJ, Heslop JP, Berridge MJ (1986). "The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues". Nature. 320 (6063): 631–4. doi:10.1038/320631a0. PMID 3010126.
- Irvine RF, Schell MJ (May 2001). "Back in the water: the return of the inositol phosphates". Nature Reviews Molecular Cell Biology. 2 (5): 327–38. doi:10.1038/35073015. PMID 11331907.