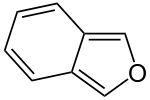
Isobenzofuran
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Benzofuran | |
| Other names
2-Oxa-2H-isoindene; Benzo[c]furan | |
| Identifiers | |
| 270-75-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:35261 |
| ChemSpider | 9553388 |
| PubChem | 11378474 |
| |
| |
| Properties | |
| C8H6O | |
| Molar mass | 118.13 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Isobenzofuran is a heterocyclic compound consisting of fused benzene and furan rings. It is isomeric with benzofuran.
Isobenzofuran is highly reactive and rapidly polymerizes; however, it has been identified[1] and prepared by thermolysis of suitable precursors and trapped at low temperature.[2]
Though isobenzofuran itself is not stable, it is the parent of related stable compounds with more complex structures.[3]
References
- ↑ Fieser, L. F.; Haddadin, M. J. (1964). "Isobenzofuran, a Transient Intermediate". Journal of the American Chemical Society. 86 (10): 2081–2082. doi:10.1021/ja01064a044.
- ↑ Wege, D. (1971). "Isolation of Isobenzofuran". Tetrahedron Letters. 12 (25): 2337–2338. doi:10.1016/S0040-4039(01)96856-X.
- ↑ Joule, J. A.; Mills, K.; Smith, G. F. (1995). Heterocyclic Chemistry (3rd ed.). CRC Press. pp. 364–365. ISBN 978-0748740697.
This article is issued from Wikipedia - version of the 6/1/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.