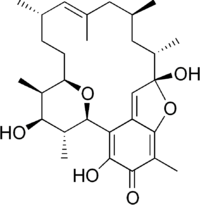
Kendomycin
 | |
| Names | |
|---|---|
| IUPAC name
(1R,9S,10S,12S,14E,16S,19R,20R,21S,22R)-3,9,21-Trihydroxy-5,10,12,14,16,20,22-heptamethyl-23,24-dioxatetracyclo[17.3.1.16,9.02,7]tetracosa-2,5,7,14-tetraen-4-one | |
| Systematic IUPAC name
(1R,9S,10S,12S,14E,16S,19R,20R,21S,22R)-3,9,21-Trihydroxy-5,10,12,14,16,20,22-heptamethyl-23,24-dioxatetracyclo[17.3.1.16,9.02,7]tetracosa-2,5,7,14-tetraen-4-one | |
| Other names
(-)-TAN 2162 | |
| Identifiers | |
| 59785-91-0 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL523927 |
| ChemSpider | 4582118 |
| MeSH | C485395 |
| PubChem | 5472093 |
| |
| |
| Properties | |
| C29H42O6 | |
| Molar mass | 486.64 g/mol |
| Appearance | Yellow powder |
| Solubility in DMSO, methanol | Soluble |
| Hazards | |
| Main hazards | Toxic |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Kendomycin is an antitumor macrolide antibiotic first isolated from the bacteria Streptomyces violaceoruber.[2] It has potent activity as an endothelin receptor antagonist and anti-osteoporosis agent.[3] It also has strong cytotoxicity against various tumor cell lines.[2]
Total synthesis
Because of its potent biological activities, kendomcyin has attracted interest as a target of total synthesis. The first total synthesis of kendomycin was accomplished by Lee and Yuan in 2004.[4] The total number of syntheses stands at 6 [5][6][7][8][9]
References
- ↑ Kendomycin at Alexis-Biochemicals
- 1 2 H B Bode; A Zeeck (2000). "Structure and biosynthesis of kendomycin, a carbocyclic ansa-compound from Streptomyces". J. Chem. Soc. Perkin Trans. 1. 323 (3): 323–328. doi:10.1039/a908387a.
- ↑ Burke Research Group University of Wisconsin
- ↑ Yu Yuan; Hongbin Men; Chulbom Lee (2004). "Total Synthesis of Kendomycin: A Macro−C−Glycosidation Approach". J. Am. Chem. Soc. 126 (45): 14720–14721. doi:10.1021/ja0447154. PMC 1785127
 . PMID 15535687.
. PMID 15535687. - ↑ A B Smith III; E F Mesaros; E Meyer (2006). "Evolution of a Total Synthesis of (−)-Kendomycin Exploiting a Petasis−Ferrier Rearrangement/Ring-Closing Olefin Metathesis Strategy". J. Am. Chem. Soc. 128 (15): 5292–9. doi:10.1021/ja060369+. PMID 16608366.
- ↑ J T Lowe; J S Panek (2008). "Total Synthesis of (−)-Kendomycin". Org. Lett. 10 (17): 3813–6. doi:10.1021/ol801499s. PMID 18698784.
- ↑ K B Bahnck; S D Rychnovsky (2008). "Formal Synthesis of (−)-Kendomycin Featuring a Prins-Cyclization to Construct the Macrocycle". J. Am. Chem. Soc. 130 (13): 177. doi:10.1021/ja805187p. PMC 2697922
 . PMID 18767844.
. PMID 18767844. - ↑ Magauer, Thomas; Martin, Harry J.; Mulzer, Johann (2009). "Total Synthesis of the Antibiotic Kendomycin by Macrocyclization using Photo-Fries Rearrangement and Ring-Closing Metathesis". Angewandte Chemie International Edition. 48 (33): 6032–6. doi:10.1002/anie.200900522. PMID 19350596.
- ↑ Martin, Harry J.; Magauer, Thomas; Mulzer, Johann (2010). "In Pursuit of a Competitive Target: Total Synthesis of the Antibiotic Kendomycin". Angewandte Chemie International Edition. 49 (33): n/a. doi:10.1002/anie.201000227. PMID 20818753.
This article is issued from Wikipedia - version of the 7/26/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.