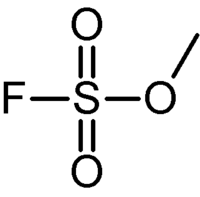
Methyl fluorosulfonate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Methyl fluorosulfonate | |
| Other names
Methyl fluorosulphonate; fluorosulfonic acid; methyl ester; methyl fluorosulphate; magic methyl | |
| Identifiers | |
| 421-20-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 9486 |
| ECHA InfoCard | 100.006.369 |
| PubChem | 9870 |
| |
| |
| Properties | |
| CH3O3FS | |
| Molar mass | 114.09 g/mol |
| Density | 1.45 g/mL |
| Boiling point | 93 °C (199 °F; 366 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Methyl fluorosulfonate, (also known as magic methyl, a name coined by George Olah) has the chemical formula F-SO2-OCH3. It is prepared by distillation of an equimolar mixture of fluorosulfonic acid and dimethyl sulfate, and used as a powerful methylating reagent (about four orders of magnitude more reactive than methyl iodide). Since it will just as readily methylate biological tissues,[1] it is acutely toxic (LC50 (rat) ~ 5 ppm), causing irritation of the airways and pulmonary edema, presumably by methylation of lipids in the cell membranes. Its use as a methylating reagent is banned by many organisations as a result.
References
- ↑ Hite, M.; Rinehart, W.; Braun, W.; Peck, H. (1979). "Acute toxicity of methyl fluorosulfonate (Magic Methyl)". AIHA Journal. 40 (7): 600–603. doi:10.1080/00028897708984416. PMID 484483.
This article is issued from Wikipedia - version of the 7/13/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.