Mambalgins
Mambalgins are peptides in the highly-potent venom of the deadly African elapid snake species Dendroaspis polylepsis, commonly known as the Black mamba. This peptide toxin was discovered by Dr. Sylvie Diochot, part of a research group directed by Dr. Eric Lingueglia from the French Mediterranean coastal city Nice, after a number of experiments carried out with venoms of a wide range of snakes.[1]
This discovery surprised the researchers because mambalgins have a potent analgesic effect,[2] which is not typical of such a deadly and dangerous reptile, given that many other snakes instead of omitting pain, produce it in great measure.[3] The researchers named the peptide by combining mamba (the name of the snake), and algin (analgesic power).
Structure
Mambalgins are classified as being part of the family of three-finger toxins.[1] This type of peptide toxin is found in snake venoms (specifically in black mambas) and is characterised by its distinctive structure.
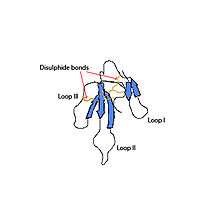
So far only two distinct isoforms of mambalgin are known; these isoforms have been given the names of mambalgin-1 and mambalgin-2. Both of these isopeptides are made of a 57 aminoacidic chain with 8 residues of cysteine. These two isoforms differ only in the residue located in the fourth position of the chain.[1]
Mambalgin-1 presents a three-dimensional structure of three loops that emerge from the nucleus of this protein. A triple chain with antiparallel β-sheets connects loops II and III, and a double chain, also formed by antiparallel β-sheets, allows the formation and bonding of loop I. Moreover, the protein presents a concave area, which is typical of neurotoxins, stabilized by four disulphide bonds.[1]
Mambalgins also show a high electrostatic potential, which is necessary for bonding with the ASIC ionic channels, which are negatively charged.

Before mambalgins were discovered, other toxins also related to the interaction with ASIC channels had already been known (PcTx1 and APETx2). Nevertheless, scientists have proved that mambalgins do not share the same amino acid sequences as these other ASIC related toxins, but are rather more similar in structure to proteins found in the venom of cobras. Specifically, the DNA sequence of mambalgins has a 55% coincidence with the sequence in proteins CM-3 and CM-2a of Naja Haje annulifera and a 47% coincidence with the CM-1b protein and the alpha-neurotoxin OH-26 of Hemachatus Haemachatus and Ophiophagus Hannah, respectively.
Properties
These polypeptide toxins found in black mamba’s venom have been proved to have a strong analgesic effect in both central and peripheral nerves, being able to be as potent as morphine but better because they cause less tolerance and no respiratory distress. While morphine acts on the opioid pathway of the brain causing addiction, headaches, difficulty thinking and vomiting, mambalgins ameliorate pain using a completely different route, which is potentially capable of causing fewer side effects.[4]
Mambalgins have been found to take away pain by inhibiting acid-sensing ion channels (ASIC) in the peripheral and central nervous system.[1] In a situation where an external pain stimulus is received by the body, the damaged cells release an ‘inflammatory soup’ of ions and other chemicals which subsequently provoke the resulting unpleasant neurological feeling of pain.[5] These ions are detected by the ASIC channels, which open up due to a change in pH levels that the ions cause. As the protein ion channels open, they communicate with the brain via an electric impulse, which the brain interprets as signifying danger or damage to the body. Mambalgins derive their pain-relieving qualities by binding to these ASIC channels and preventing them from opening and thus blocking pain perception.[5] Nevertheless, it is important to bear in mind that mambalgins do not block all ion channels, because these kinds of toxins are normally highly specific. Researchers have found that mambalgins have a potent, rapid and reversible effect in homomeric ASIC1 and heteromeric ASIC1a+ASIC2a or ASIC1a+ASIC2b channels, which are the entire ASIC channel subtypes found in the central nervous system. On the other hand, they have no effect on ASIC2a, ASIC3, ASIC1a+ASIC3 and ASIC1b+ASIC3 channels.[1]
Applications
As has been commented previously, a group of researchers of Niza (France) discovered, after analysing 50 different toxins of various snake venoms, a type of proteins named mambalgins, found in the venom of the African Black Mamba.
After an experimental study carried out on mice, the results obtained indicated that mambalgins have analgesic properties, with the advantage that they don’t provoke the characteristic secondary effects of morphine.[6] In this study, a group of mice treated with mambalgins could withstand hot water applied in their tails and paws for much more time than those that were not treated with the protein.[6] What’s more, five days later, mice treated with morphine had generated tolerance towards opioids—typical of this type of drug. Conversely, in the mambalgin-treated mice, the effect of this tolerance was less noticeable.[6]
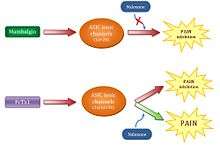
Thanks to further experiments carried out by the team directed by Dr. Silvie Diochot, it has been observed that mambalgins are a very good alternative to morphine as an analgesic. This is because they have a great power to inhibit pain and have also the distinctive feature of being able to avoid pain without the implication of side effects that other opioid analgesics such as morphine entail. It has also been shown that they follow a different pathway affecting the brain, which explains why the administration of the antagonistic opioid naloxone doesn’t inhibit its effect.
Bringing mambalgins to a practical use, these peptides could be useful in the analgesic treatments of patients with chronic respiratory diseases, given that their effect seems not to affect the respiratory system, in contrast to morphine which can cause respiratory distress. In addition, this new potential drug seems to deal with the problem of tolerance using morphine, in other words, needing an increasingly higher dose each time to obtain the same effect. This would be of great use for the chronically ill, as they could take the analgesic as many times as they wanted without becoming addicts or dependant on the drug. Other side effects such as constipation could also be avoided by using mambalgins instead of morphine.
Even though mambalgins affect the brain through a different pathway compared to morphine, this property could be positively used in order to develop a new research area, consisting in the exploration of whether these peptides could play an important role in the coming off of drugs in addicted to opioids patients such as heroin addicts. Nevertheless, there is much research left to do regarding the effects that these drugs could have on human patients, seeing if mambalgins are actually a better alternative to counteract the secondary effects of many opioids, with the same capacity of blocking pain.
See also
References
- 1 2 3 4 5 6 Nature. "Black mamba venom peptides target acid-sensing ion channels to abolish pain".
- ↑ Nature. "Black mamba takes away pain"
- ↑ Nature. "A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain."
- ↑ BBC.'Black mamba venom is better painkiller than morphine'
- 1 2 Discover magazine. 'Painkilling chemicals with no side effects found in black mamba venom'
- 1 2 3 Muy interesante. 'Veneno de serpiente para el dolor'