Temporal lobe epilepsy
| Temporal lobe epilepsy (TLE) | |
|---|---|
 | |
| Lobes of the brain, temporal lobe in pink | |
| Classification and external resources | |
| Specialty | neurology |
| ICD-10 | G40.1-G40.2 |
| ICD-9-CM | 345.4 |
| DiseasesDB | 29433 |
| MedlinePlus | 001399 |
| eMedicine | neuro/365 |
| Patient UK | Temporal lobe epilepsy |
| MeSH | D004833 |
Temporal lobe epilepsy (TLE) is a chronic disorder of the nervous system characterized by recurrent, unprovoked focal seizures (also known as partial seizures) that originate in the temporal lobe of the brain and last about one or two minutes. TLE is the most common form of epilepsy with focal seizures.[1]
People with TLE may experience simple partial seizures that only affect the temporal lobe or complex partial seizures that spread to other regions of the brain. During simple partial seizures, some people remain conscious. Depending on the areas of the brain affected, people with TLE may experience chest discomfort, nausea, unexplained emotions (e.g., intense joy or fear), or loss of awareness (e.g., staring or repetitive behaviors like blinking, twitching, pacing, etc.). They might also be in a dream-like state and have changes in consciousness, strange sensations, or have hallucinations and see, hear, feel, smell, or taste things that are not real. Some people report auras (warnings that a seizure is approaching), usually described as intense feelings of déjà vu or fear. Usually auras are actually the focal seizure itself, but some people do develop a regular progression of symptoms before each seizure that can serve as a warning.[1]
TLE is usually diagnosed in childhood or by the teenage years. Physicians diagnose TLE by taking a medical history, blood tests, and brain imaging (EEG, CT scan, PET, and/or MRI). It can have a number of causes such as head injury, stroke, brain infections, structural lesions in the brain, or brain tumors, or it can be idiopathic and have no apparent cause. The first line of treatment is through anticonvulsant medication. Surgery may be an option for some people, especially when there is an observable abnormality in the brain. Another treatment option is electrical stimulation of the brain through an implantation called the vagus nerve stimulation (VNS) device.[1]
Types
Over forty types of epilepsy are recognized and these are divided into two main seizures: Partial seizure and generalized seizure. Partial seizures account for approximately sixty percent of all adult cases.[2] Temporal lobe epilepsy (TLE) is the single most common form of partial seizure.[3]
The International League Against Epilepsy (ILAE) recognizes two main types of temporal lobe epilepsy: mesial temporal lobe epilepsy (MTLE), arising in the hippocampus, the parahippocampal gyrus and the amygdala which are located in the inner (medial) aspect of the temporal lobe and lateral temporal lobe epilepsy (LTLE), the rarer type, arising in the neocortex at the outer (lateral) surface of the temporal lobe.[2] The seizures of LTLE are characterized by auditory or visual features. Autosomal dominant Lateral Temporal Lobe Epilepsy (ADLTLE) is a rare hereditary condition.
In 2013, the ILAE published a new classification of MTLE based on abnormalities (hippocampal sclerosis) found at the microscopic level in the tissue of the hippocampus.[4] However, the pathology changes used in the classification cannot easily be predicted by clinical features.[5]
Signs and symptoms
When a seizure begins in the temporal lobe, its symptoms and the patient's behavior as seen by bystanders, depend on the precise location of its point of origin, its locus. In 1981, the ILAE recognized three types of seizures occurring in temporal lobe epilepsy. The classification was based on EEG findings.[6]
Simple partial seizures
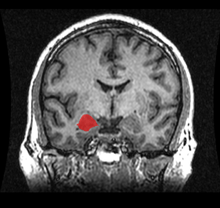
Simple partial seizures (SPS) involve small areas of the temporal lobe such as the amygdala and hippocampus. The term "simple" means that the level of consciousness of the patient is not altered during the seizure. In temporal lobe epilepsy, a simple partial seizure usually causes abnormal sensations only.
These may be mnestic sensations such as déjà vu (a feeling of familiarity), jamais vu (a feeling of unfamiliarity); amnesia; or a single memory or set of memories; auditory (an abnormal sound or tune); gustatory (an abnormal taste); olfactory (a smell that is not physically present); visual; or sensory (involving feelings on the skin or in the internal organs) sensations. Sensory disturbances may seem to move over the body. Synesthesia (stimulation of one sense experienced in a second sense) may transpire.[7] Dysphoric or euphoric feelings, fear, anger, and other emotions may also occur. Often, the patient cannot describe the sensations.
Simple partial seizures may be called "auras" by lay people who mistake them for a warning sign of a subsequent seizure. Instead, the so-called aura is actually the partial seizure itself. People who only experience simple partial seizures may not recognize what they are, nor seek medical advice about them. A simple partial seizure may or may not progress to any of the seizure types listed below.[8]
Complex partial seizures
Complex partial seizures (CPS) are seizures which impair consciousness to some extent: they alter the person's ability to interact normally with their environment. They usually begin with a simple partial seizure, then spread to a larger portion of the temporal lobe, resulting in impaired consciousness. Signs may include motionless staring, automatic movements of the hands or mouth, altered ability to respond to others, unusual speech, or other unusual behaviors.[9]
These seizures tend to have a warning or aura before they occur, and when they occur they generally tend to last only 1–2 minutes. It is not uncommon for an individual to be tired or confused for up to 15 minutes after a seizure has occurred. Though they may not seem harmful, due to the fact that the individual does not normally seize, they can be extremely harmful if the individual is left alone around dangerous objects. For example, if a person with complex partial seizures is driving alone, this can cause them to run into the ditch, or worse cause an accident involving multiple people. With this type, some people do not even realize they are having a seizure and most of the time their memory from right before or after the seizure is wiped. First-aid is only required if there has been an injury or if this is the first time a person has had a seizure.
Secondarily generalized tonic-clonic seizures
Seizures which begin in the temporal lobe, and then spread to involve the whole brain are known as Secondarily Generalized Tonic-Clonic Seizures (SGTCS). The arms, trunk, and legs stiffen (the tonic phase), in either a flexed or extended position, and then jerk (the clonic phase). Secondarily generalized tonic clonic seizures are known in the vernacular as convulsions or grand mal seizures.[9] The word grand mal comes from the French term, meaning major affliction.
Postictal period
There is some period of recovery in which neurological function is altered after each of these seizure types. This is the postictal state. The degree and length of postictal impairment directly correlates with the severity of the seizure type. Simple partial seizures often last less than sixty seconds; complex partial seizures may last up to two minutes; and generalized tonic clonic seizures may last up to three minutes. The postictal state in the case of complex partial seizures and generalised tonic clonic seizures, the postictal state may last much longer than the seizure itself.
Because a major function of the temporal lobe is short-term memory, complex partial seizures and generalised tonic clonic seizures may cause amnesia for the period of the seizure. As a result, patients with temporal lobe complex partial seizures and generalised tonic clonic seizures may not remember having had a seizure.
Interictal period
Investigations which examine cerebral blood flow show a reduction in the perfusion of the ipsilateral temporal lobe between seizures in patients with intractable TLE. These investigations include H(2)(15)O positron emission tomography (PET) scanning and pulsed arterial spin labeling MR imaging.[10]
Causes
The causes of TLE include mesial temporal sclerosis, traumatic brain injury, brain infections, such as encephalitis and meningitis, hypoxic brain injury, stroke, cerebral tumours, and genetic syndromes. Temporal lobe epilepsy is not the result of mental health disorders or fragility of the personality.[9]
Febrile seizures
Although the theory is controversial, there is a link between febrile seizures (seizures coinciding with episodes of fever in young children) and subsequent temporal lobe epilepsy, at least epidemiologically.[11][12][13][14]
Human herpes virus 6
In the mid 1980s, human herpesvirus 6 (HHV-6) was suggested as a possible causal link between febrile convulsions and mesial temporal lobe epilepsy. However, although the virus is found in temporal lobe tissue at surgery for TLE, it has not been recognised as a major factor in febrile seizures or TLE.[15][16][17]
Reelin
Dispersion of the granule cell layer in the hippocampal dentate gyrus is occasionally seen in temporal lobe epilepsy and has been linked to the downregulation of reelin, a protein that normally keeps the layer compact by containing neuronal migration. It is unknown whether changes in reelin expression play a role in epilepsy.[18][19]
Pathophysiology
Neuronal loss
In TLE, there is loss of neurons in region CA1 and CA3 of the hippocampus.[20][21] There is also damage to mossy cells and inhibitory interneurons in the hilar region of the hippocampus (region IV) and to the granule cells of the dentate gyrus. In animal models, neuronal loss occurs during seizures but in humans, neuronal loss predates the first seizure and does not necessarily continue with seizure activity.[22][23][24][25][26] The loss of the GABA-mediated inhibitory interneurons may increase the hyperexcitability of neurons of the hippocampus leading to recurrent seizures.[27] According to the "dormant basket cell" hypothesis, mossy cells normally excite basket cells which in turn, inhibit granule cells. Loss of mossy cells lowers the threshold of action potentials of the granule cells.[28]
Granule cell dispersion in the dentate gyrus
Granule cell dispersion is a type of developmental migration and a pathological change found in the TLE brain which was first described in 1990.[29][30] The granule cells of the dentate gyrus are tightly packed forming a uniform, laminated layer with no monosynaptic connections.[31] This structure provides a filter for the excitability of neurons.[31]
In TLE, granule cells are lost, the structure is no longer closely packed and there are changes in the orientation of dendrites.[30][32] These changes may or may not be epileptogenic. For instance, if the dendrites of granule cells reconnect, it may be in a way (through the laminar planes) that allows hyperexcitability.[23] However, not all patients have granule cell dispersion.[20](p387–389)
Aberrant mossy fiber sprouting
Mossy fibers are the axons of Ponte nuclei cells. They project into the hilum of the dentate gyrus and stratum lucidum in the CA3 region giving input to both excitatory and inhibitory neurons.[31][33][34]
In the TLE brain, where granule cells are damaged or lost, axons, the mossy fibres, 'sprout' in order to reconnect to other granule cell dendrites. This is an example of synaptic reorganisation. This was noted in human tissue in 1974 and in animal models in 1985. In TLE, the sprouting mossy fibres are larger than in the normal brain and their connections may be aberrant. Mossy fibre sprouting continues from one week to two months after injury.[20](p416–431)[31][35][36][37]
Aberrant mossy fibre sprouting may create excitatory feedback circuits that lead to temporal lobe seizures. This is evident in intracellular recordings.[38] Stimulation of aberrant mossy fibre areas increases the excitatory postsynaptic potential response.[39][40]
However, aberrant mossy fiber sprouting may inhibit excitatory transmission by synapsing with basket cells which are inhibitory neurons and by releasing GABA and neuropeptide Y which are inhibitory neurotransmitters. Also, in animal models, granule cell hyper-excitability is recorded before aberrant mossy fibre sprouting has occurred.[41][42][43][44]
Diagnosis
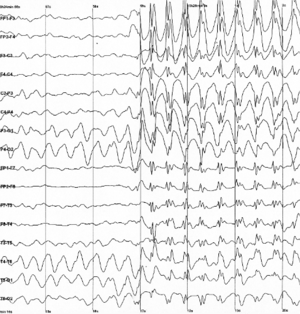
Temporal lobe epilepsy diagnosis can be done via the following methods/exams:[45]
Differential diagnosis
Among the DDx to look out for are panic attacks, tardive dyskinesia, occipital lobe epilepsy and psychogenic seizures (though this is not an exhaustive list)[46]
Treatments
Antiepileptic drugs
There are many oral medications available for the management of epileptic seizures. They were previously called anticonvulsants. However, this term is misleading because most TLE seizures are, in fact, not convulsions. The modern term is antiepileptic drugs (AEDs). Nearly all AEDs function by decreasing the excitation of neurons, for example, by blocking fast or slow sodium channels or by modulating calcium channels; or by enhancing the inhibition of neurons, for example by potentiating the effects of inhibitory neurotransmitters like GABA.
In TLE, the most commonly used older AEDs are phenytoin, carbamazepine, primidone, valproate, and phenobarbital. Newer drugs, such as gabapentin, topiramate, levetiracetam, lamotrigine, pregabalin, tiagabine, lacosamide, and zonisamide promise similar effectiveness, with possibly fewer side-effects. Felbamate and vigabatrin are newer AEDs, but can have serious adverse effects so they are not considered first-line treatments.
Up to one third of patients with medial temporal lobe epilepsy will not have adequate seizure control with medication alone. For patients with medial TLE whose seizures remain uncontrolled after trials of several AEDs (that is, the epilepsy is intractable), surgical excision of the affected temporal lobe may be considered.[47]
Surgical interventions
Epilepsy surgery has been performed since the 1860s and doctors have observed that it is highly effective in producing freedom from seizures. However, it was not until 2001 that a scientifically sound study was carried out to examine the effectiveness of temporal lobectomy.[48]
Temporal lobe surgery can be complicated by decreased cognitive function. However, after temporal lobectomy, memory function is supported by the opposite temporal lobe; and recruitment of the frontal lobe.[49][50] Cognitive rehabilitation may also help.[51]
Other treatments
If a person is not an optimal candidate for epilepsy surgery, further management options include, new (including experimental) antiepileptic drugs, vagus nerve stimulation, and in children, the ketogenic diet. Other avenues of treatment include brain cortex responsive neural stimulators, deep brain stimulation, stereotactic radiosurgery, such as the gamma knife, and stereotactic laser ablation.[52]
Complications and prognosis
Childhood onset
After childhood onset, one third will "grow out" of TLE, finding a lasting remission up to an average of 20 years. The finding of a lesion such as hippocampal sclerosis (a scar in the hippocampus), tumour, or dysplasia, on magnetic resonance imaging (MRI) predicts the intractability of seizures.[53]
Personality
The effect of temporal lobe epilepsy on personality is an historical observation dating to the 1800s. Sigmund Freud said
- "We know that epilepsy produces these remarkable changes in the personality."[54]
Personality and behavioural change in temporal lobe epilepsy is seen as a chronic condition when it persists for more than three months. A particular neural location (especially on the surface of the temporal lobe) may be the origin of such changes.[55]
Depression
Individuals with temporal lobe epilepsy have a high prevalence of depression. Although the psychosocial impacts of epilepsy may be causative, there are also links in the phenomenology and neurobiology of TLE and depression.[56]
Psychosis
Psychosis may occur in the peri-ictal, post-ictal, and inter-ictal periods in TLE.[57]
Memory
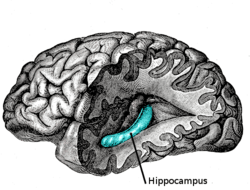
The temporal lobe and particularly the hippocampus plays an important role in memory processing. Declarative memory (memories which can be consciously recalled) is formed in the area of the hippocampus called the dentate gyrus.
Temporal lobe epilepsy is associated with memory disorders and loss of memory. Animal models and clinical studies show that memory loss correlates with temporal lobe neuronal loss in temporal lobe epilepsy. Verbal memory deficit correlates with pyramidal cell loss in TLE. This is more so on the left in verbal memory loss. Neuronal loss on the right is more prominent in non-verbal (visuospatial memory loss).[58][59][60][61][62]
Link with religiosity
The first to record and catalog the abnormal symptoms and signs of TLE was Norman Geschwind. He found a constellation of symptoms that included hypergraphia, hyperreligiosity, collapse, and pedantism, now called Geschwind syndrome.
Vilayanur S. Ramachandran explored the neural basis of the hyperreligiosity seen in TLE using the galvanic skin response (GSR), which correlates with emotional arousal, to determine whether the hyperreligiosity seen in TLE was due to an overall heightened emotional state or was specific to religious stimuli. Ramachandran presented two subjects with neutral, sexually arousing and religious words while measuring GSR. Ramachandran was able to show that patients with TLE showed enhanced emotional responses to the religious words, diminished responses to the sexually charged words, and normal responses to the neutral words. This study was presented as an abstract at a neuroscience conference and referenced in Ramachandran's book, Phantoms in the Brain,[63] but it has never been published in the peer-reviewed scientific press.[64]
One recent study reported that intrinsic religiosity and religiosity outside of organized religion were higher in patients with epilepsy than in controls.[65] Lower education level, abnormal background EEG activity, and hippocampal sclerosis have been found to be contributing factors for religiosity in Temporal Lobe Epilepsy.[66]
Temporal lobe epilepsy has been suggested as a physical explanation for the revelatory experiences of prominent religious figures such as Abraham, Moses, Jesus, Mohammed, Saint Paul, and Joseph Smith. These experiences are described as complex interactions with their visions, but lacking the stereotypy, amnestic periods, and automatisms or generalized motor events, which are characteristic of TLE. Psychiatric conditions with psychotic spectrum symptoms are a more plausible physical explanation of these experiences.[67] Pope Pius IX's doctrine of the immaculate conception is thought to have been influenced by his forensically diagnosed partial epilepsy.[68] It has also been suggested that the visions of Joan of Arc were probably an expression of partial epilepsy.[69] More recently, one case history found that a temporal lobe epileptic experienced a vision of God following a temporal lobe seizure, while undergoing EEG monitoring. The patient reported that God had sent him to the world to "bring redemption to the people of Israel".[70]The purported link between TLE and religiosity has inspired work by Michael Persinger and many other researchers in the field of neurotheology, but some have questioned the evidence for a link between temporal lobe epilepsy and religiosity.[64][71] The novel, Lying Awake, by Mark Salzman, deals with topic of temporal lobe epilepsy and religion.
See also
References
- 1 2 3 NINDS (1 February 2016), The Epilepsies and Seizures: Hope Through Research, National Institute of Neurological Disorders and Stroke (NINDS), U.S. National Institutes of Health (NIH), retrieved 8 August 2016
- 1 2 Engel J (2001). "A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology". Epilepsia. 42 (6): 796–803. doi:10.1046/j.152a8-1157.2001.10401.x. PMID 11422340.
- ↑ Wiebe S (May 2000). "Epidemiology of temporal lobe epilepsy". Can J Neurol Sci. 27 Suppl 1: S6–10; discussion S20–1. doi:10.1017/s0317167100000561. PMID 10830320.
- ↑ Blumke I, et al. (2013). "International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a task force report from the ILAE commission on diagnostic methods". Epilepsia. 54 (7): 1315–1329. doi:10.1111/epi.12220. PMID 23692496.
- ↑ Fauser S, et al. (2013). "Is there evidence for clinical differences related to the new classification of temporal lobe cortical dysplasia?". Epilepsia. 54 (5): 909–917. doi:10.1111/epi.12147. PMID 23551067.
- ↑ ILAE (1981). "Proposal for revised clinical and electroencephalographic classification of epileptic seizures". Epilepsia. 22: 489–501. doi:10.1111/j.1528-1157.1981.tb06159.x. PMID 6790275.
- ↑ Marcel Neckar; Petr Bob (11 January 2016). "Synesthetic associations and psychosensory symptoms of temporal epilepsy". Neuropsychiatric Disease and Treatment. National Institutes of Health (NIH). Retrieved 21 April 2016.
- ↑ "Simple partial seizures." Johns Hopkins Medicine website. Accessed 2 February 2014.
- 1 2 3 "Temporal lobe epilepsy." Mayo Clinic patient information website. Accessed 2 February 2014.
- ↑ Lim Y. M.; et al. (2008). "Usefulness of pulsed arterial spin labeling MR imaging in mesial temporal lobe epilepsy". Epilepsy Res. 82 (2-3): 183–189. doi:10.1016/j.eplepsyres.2008.08.001. PMC 2597620
 . PMID 19041041.
. PMID 19041041. - ↑ Shinnar S, et al. (2008). "Phenomenology of prolonged febrile seizures: results of the FEBSTAT study". Neurology. 71 (3): 170–176. doi:10.1212/01.wnl.0000310774.01185.97. PMID 18525033.
- ↑ Tarkka R, et al. (2003). "Febrile seizures and mesial temporal sclerosis: no association in a long-term follow-up study". Neurology. 60 (2): 215–218. doi:10.1212/01.WNL.0000037482.55894.B1. PMID 12552033.
- ↑ Berg A. T.; et al. (1999). "Childhood-onset epilepsy with and without preceding febrile seizures". Neurology. 53 (8): 1742–1748. doi:10.1212/WNL.53.8.1742. PMID 10563622.
- ↑ Provenzale J. M.; et al. (2008). "Hippocampal MRI signal hyperintensity after febrile status epilepticus is predictive of subsequent mesial temporal sclerosis". Am J Roentgenol. 190 (4): 976–983. doi:10.2214/AJR.07.2407. PMID 18356445.
- ↑ Karatas H, Gurer G, Pinar A, et al. (January 2008). "Investigation of HSV-1, HSV-2, CMV, HHV-6 and HHV-8 DNA by real-time PCR in surgical resection materials of epilepsy patients with mesial temporal lobe sclerosis". J. Neurol. Sci. 264: 151–6. doi:10.1016/j.jns.2007.08.010. PMID 17804017.
- ↑ Fotheringham J.; et al. (2007). "Association of Human Herpesvirus-6B with Mesial Temporal Lobe Epilepsy". PLoS Med. 4 (5): e180. doi:10.1371/journal.pmed.0040180. PMC 1880851
 . PMID 17535102.
. PMID 17535102. - ↑ Donati D, et al. (2003). "Detection of human herpesvirus-6 in mesial temporal lobe epilepsy surgical brain resections". Neurology. 61 (10): 1405–1411. doi:10.1212/01.WNL.0000094357.10782.F9. PMID 14638964.
- ↑ Haas C. A.; et al. (2002). "Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy". J. Neurosci. 22 (14): 5797–6802. PMID 12122039.
- ↑ Heinrich C, et al. (2006). "Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus". J. Neurosci. 26 (17): 4701–4713. doi:10.1523/JNEUROSCI.5516-05.2006. PMID 16641251.
- 1 2 3 de Lanerolle N. C. and Noebels J. L. (ed.) Jasper's basic mechanisms of the epilepsies: histopathology of human epilepsy. Oxford University Press 2012 chapter 30 ISBN 978-0-19-974654-5.(p387–389)
- ↑ Liu Z, et al. (1994). "Quantitative evaluation of neuronal loss in the dorsal hippocampus in rats with long-term pilocarpine seizures". Epilepsy Research. 17 (3): 237–247. doi:10.1016/0920-1211(94)90054-x. PMID 8013446.
- ↑ Blümcke I, et al. (2000). "Loss of hilar mossy cells in Ammon's horn sclerosis". Epilepsia. 40 (6): 174–180. doi:10.1111/j.1528-1157.2000.tb01577.x. PMID 10999540.
- 1 2 Sloviter R. S. (1987). "Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy". Science. 235 (4784): 73–76. doi:10.1126/science.2879352. PMID 2879352.
- ↑ Kobayashi M.; Buckmaster P. S (2003). "Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy". Journal of Neuroscience. 23 (6): 2440–2452. PMID 12657704.
- ↑ Bouilleret V, et al. (1999). "Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy". Neuroscience. 89 (3): 717–729. doi:10.1016/s0306-4522(98)00401-1. PMID 10199607.
- ↑ Thorn M, et al. (2005). "Quantitative post-mortem study of the hippocampus in chronic epilepsy: seizures do not inevitably cause neuronal loss". Brain. 128 (6): 1344–1357. doi:10.1093/brain/awh475. PMID 15758032.
- ↑ Meldrum B (1989). "GABAergic mechanisms in the pathogenesis and treatment of epilepsy". British Journal of Clinical Pharmacology. 1: 3–11. doi:10.1111/j.1365-2125.1989.tb03454.x. PMID 2667605.
- ↑ Sloviter R. S.; et al. (2003). "Dormant basket cell" hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat". Journal of Comparative Neurology. 459 (1): 44–76. doi:10.1002/cne.10630. PMID 12629666.
- ↑ Thom M, et al. (2005). "Cell proliferation and granule cell dispersion in human hippocampal sclerosis". J Neuropathol Exp Neurol. 64 (3): 194–201. PMID 15804050.
- 1 2 Houser C. R. (1990). "Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy". Brain Research. 535 (2): 195–204. doi:10.1016/0006-8993(90)91601-c. PMID 1705855.
- 1 2 3 4 Nadler J. V. (2003). "The recurrent mossy fiber pathway of the epileptic brain". Neurochemical Research. 28 (11): 1649–1658. PMID 14584819.
- ↑ Freimanr T. M.; et al. (2011). "Granule cell dispersion in temporal lobe epilepsy is associated with changes in dendritic orientation and spine distribution". Experimental Neurology. 229 (2): 332–338. doi:10.1016/j.expneurol.2011.02.017. PMID 21376037.
- ↑ Lim C, et al. (1997). "Connections of the hippocampal formation in humans: I. The mossy fiber pathway". Journal of Comparative Neurology. 385 (3): 325–351. doi:10.1002/(sici)1096-9861(19970901)385:3<325::aid-cne1>3.0.co;2-5. PMID 9300763.
- ↑ Henze D. A.; et al. (2000). "The multifarious hippocampal mossy fiber pathway: a review". Neuroscience. 98 (3): 407–427. doi:10.1016/s0306-4522(00)00146-9. PMID 10869836.
- ↑ Scheibel P, et al. (1974). "The hippocampal-dentate complex in temporal lobe epilepsy". Epilepsia. 15 (1): 55–80. doi:10.1111/j.1528-1157.1974.tb03997.x. PMID 4523024.
- ↑ Steward O. "Lesion-induced synapse reorganization in the hippocampus of cats: sprouting of entorhinal, commissural/associational, and mossy fiber projections after unilateral entorhinal cortex lesions, with comments on the normal organization of these pathways." Hippocampus 1992 2(3) pp. 247-268 PMID 1284974 doi:10.1002/hipo.450020305
- ↑ Babb T. L.; et al. (1991). "Synaptic reorganization by mossy fibers in human epileptic fascia dentata". Neuroscience. 42 (2): 351–363. doi:10.1016/0306-4522(91)90380-7. PMID 1716744.
- ↑ Buckmaster P, et al. (2002). "Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit". Journal of Neuroscience. 22 (15): 6650–6658. PMID 12151544.
- ↑ Nadler J. V.; et al. (1985). "Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats". Journal of Neuroscience. 5 (4): 1016–1022. PMID 3981241.
- ↑ Scharfman H. E.; et al. (2003). "Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sprouting". Journal of Neurophysiology. 90 (4): 2536–2547. doi:10.1152/jn.00251.2003. PMID 14534276.
- ↑ Sloviter R. S.; et al. (2006). "Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: possible anatomical substrate of granule cell hyperinhibition in chronically epileptic rats". Journal of Comparative Neurology. 494 (6): 960. doi:10.1002/cne.20850. PMID 16385488.
- ↑ Sloviter R. S.; et al. (2003). "Dormant basket cell hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat". Journal of Comparative Neurology. 459 (1): 44–76. doi:10.1002/cne.10630. PMID 12629666.
- ↑ Tu B, et al. (2005). "Spontaneous release of neuropeptide Y tonically inhibits recurrent mossy fiber synaptic transmission in epileptic brain". Journal of Neuroscience. 25 (7): 1718–1729. doi:10.1523/JNEUROSCI.4835-04.2005. PMID 15716408.
- ↑ Schwarzer C.; Sperk G. (1995). "Hippocampal granule cells express glutamic acid decarboxylase-67 after limbic seizures in the rat". Neuroscience. 69 (3): 705–709. doi:10.1016/0306-4522(95)00348-m. PMID 8596641.
- ↑ "Temporal Lobe Epilepsy Workup: Approach Considerations, Computed Tomography Scanning, Magnetic Resonance Imaging". emedicine.medscape.com. Retrieved 2016-08-24.
- ↑ "Temporal Lobe Epilepsy; TLE medical Information Page | Patient". Patient. Retrieved 24 August 2016.
- ↑ Kwan P (2000). "Early identification of refractory epilepsy". NEJM. 342 (5): 314–319. doi:10.1056/NEJM200002033420503. PMID 10660394.
- ↑ Wiebe S, et al. (2001). "A randomized, controlled trial of surgery for temporal lobe epilepsy". NEJM. 345 (5): 311–318. doi:10.1056/NEJM200108023450501. PMID 11484687.
- ↑ Cheung; et al. (2009). "Pre- and postoperative fMRI and clinical memory performance in temporal lobe epilepsy". J Neurol Neurosurg Psychiatry. 80 (10): 1099–1106. doi:10.1136/jnnp.2009.173161. PMID 19389718.
- ↑ Maccotta L, et al. (2007). "Changing frontal contributions to memory before and after medial temporal lobectomy". Cereb Cortex. 17 (2): 443–456. doi:10.1093/cercor/bhj161. PMID 16547345.
- ↑ Helmstaedter C, et al. (2008). "The effects of cognitive rehabilitation on memory outcome after temporal lobe epilepsy surgery". Epilepsy Behav. 12 (3): 402–409. doi:10.1016/j.yebeh.2007.11.010. PMID 18155965.
- ↑ http://www.epilepsybehavior.com/article/S1525-5050(12)00362-9/abstract?cc=y=
- ↑ Spooner C. G. (2006). "New-onset temporal lobe epilepsy in children: lesion on MRI predicts poor seizure outcome". Neurology. 67 (12): 2147–2153. doi:10.1212/01.wnl.0000248189.93630.4f. PMID 17082466.
- ↑ Registration required to view.
- ↑ Geschwind N. Registration required to view.
- ↑ Butler T, et al. (2012). "Cortical thickness abnormalities associated with depressive symptoms in temporal lobe epilepsy". Epileps Behav. 23 (1): 64–67. doi:10.1016/j.yebeh.2011.10.001. PMC 3259282
 . PMID 22099527.
. PMID 22099527. - ↑ Engel J. (2008) Epilepsy: A Comprehensive Textbook Lippincott Williams & Wilkins vol 3 ISBN 0781757770, 9780781757775. p 2115
- ↑ Sutula T, et al. (2002). "Repeated brief seizures induce progressive hippocampal neuron loss and memory deficits". Progress in Brain Research. 135: 95–110. doi:10.1016/S0079-6123(02)35010-6. PMID 12143373.
- ↑ Sass K. J.; et al. (1992). "Specificity in the correlation of verbal memory and hippocampal neuron loss: dissociation of memory, language, and verbal intellectual ability". Journal of Clinical & Experimental Neuropsychology. 41 (5): 662–672. doi:10.1080/01688639208402854. PMID 1474137.
- ↑ Babb T, et al. (1993). "Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy". Archives of Neurology. 50 (8): 812–817. doi:10.1001/archneur.1993.00540080023008. PMID 8352666.
- ↑ Sass K. J.; et al. (1990). "Verbal memory impairment correlates with hippocampal pyramidal cell density". Neurology. 40 (11): 1694–1697. doi:10.1212/wnl.40.11.1694. PMID 2234424.
- ↑ Reminger S. L.; et al. (2004). "Bilateral hippocampal volume predicts verbal memory function in temporal lobe epilepsy". Epilepsy & Behavior. 5 (5): 687–695. doi:10.1016/j.yebeh.2004.06.006. PMID 15380120.
- ↑ Ramachandran, V. and Blakeslee (1998). Phantoms in the Brain.
- 1 2 Craig Aaen-Stockdale (2012). "Neuroscience for the Soul". The Psychologist. 25 (7): 520–523.
- ↑ Tedrus, Glória Maria Almeida Souza; Fonseca, Lineu Corrêa; Fagundes, Tatiane Mariani; da Silva, Gabriela Leopoldino (2015). "Religiosity aspects in patients with epilepsy.". Epilepsy & behavior : E&B. 50: 67–70. doi:10.1016/j.yebeh.2015.06.003. PMID 26133113.
- ↑ Tedrus, Glória Maria Almeida Souza; Fonseca, Lineu Corrêa; Höehr, Gabriela Chaves (2013). "Spirituality aspects in patients with epilepsy.". Seizure. 23 (1): 25–8. doi:10.1016/j.seizure.2013.09.005. PMID 24094727.
Patients with mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS) had significantly higher SSRS scores than those with other epileptic syndromes and, than in individuals of the CG (control Group)
- ↑ Murray E. D.; et al. (2012). "The role of psychotic disorders in religious history considered". J Neuropsychiatry Clin Neuroscience. 24 (4): 410–426. doi:10.1176/appi.neuropsych.11090214. PMID 23224447.
- ↑ Sirven, Joseph I; Drazkowski, Joseph F; Noe, Katherine H (2007). "Seizures among public figures: lessons learned from the epilepsy of Pope Pius IX.". Mayo Clinic Proceedings. 82 (12): 1535–1540. doi:10.1016/S0025-6196(11)61100-2. PMID 18053463.
- ↑ d'Orsi, Giuseppe; Tinuper, Paolo (2006). ""I heard voices...": from semiology, a historical review, and a new hypothesis on the presumed epilepsy of Joan of Arc.". Epilepsy & behavior. 9 (1): 152–157. doi:10.1016/j.yebeh.2006.04.020. PMID 16750938.
- ↑ Arzy, Shahar; Schurr, Roey (2016). ""God has sent me to you": Right temporal epilepsy, left prefrontal psychosis.". Epilepsy & behavior. 60: 7–10. doi:10.1016/j.yebeh.2016.04.022. PMID 27176877. Lay summary.
In this patient, a messianic revelation experience occurred several hours after a complex partial seizure of temporal origin, compatible with postictal psychosis (PIP)
- ↑ Benson, D.F. & Hermann, B.P. (1998) Personality disorders. In J. Engel Jr. & T.A. Pedley (Eds.) Epilepsy: A comprehensive textbook. Vol. II (pp. 2065–2070). Philadelphia: Lippincott–Raven.