Methyl lactate
 | |
| Names | |
|---|---|
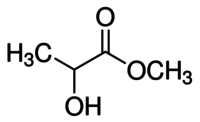
| IUPAC name
Methyl 2-hydroxypropanoate | |
| Other names
2-Hydroxy- Methyl Ester Propanoic Acid; 2-Hydroxypropanoic Acid, Methyl Ester; Lactic Acid, Methyl Ester | |
| Identifiers | |
| 547-64-8 (racemate)(L-isomer 27871-49-4 (L-isomer) 17392-83-5 (D-isomer) | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 13839235 |
| ECHA InfoCard | 100.008.119 |
| PubChem | 11040 |
| RTECS number | OD5670000 |
| |
| |
| Properties | |
| C4H8O3 | |
| Molar mass | 104.11 g·mol−1 |
| Appearance | colourless clear liquid |
| Density | 1.093 g/cm3 |
| Melting point | −66 °C (−87 °F; 207 K) |
| Boiling point | 144 to 145 °C (291 to 293 °F; 417 to 418 K) |
| Miscible | |
| Solubility in ethanol and most alcohols |
Miscible |
| Hazards | |
| Main hazards | Irritant (Xi) |
| Safety data sheet | MSDS |
| EU classification (DSD) |
|
| NFPA 704 | |
| Flash point | 49 °C (120 °F; 322 K) |
| Related compounds | |
| Related compounds |
Lactic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Methyl lactate, also known as lactic acid methyl ester, is a monobasic ester formed from lactic acid and methanol, commonly used as a solvent. Methyl lactate belongs to the lactate ester group of compounds that are considered nontoxic and highly and readily biodegradable.[1]
Methyl lactate is a colorless clear liquid, completely miscible with water and most organic liquids. It is considered as a green solvent as it is easily biodegradable. It is a solvent for nitrocellulose, cellulase acetate, cellulase acetobutyrote and cellulase acetaprapionate. It is used in the manufacture of lacquers and dopes where it contributes high tolerance for diluents, good flaw and blush resistance.[2]
Methyl lactate hydrolyzes in the presence of water and acids or bases into lactic acid and methanol.
References
- ↑ Aparicio, Santiago (May 10, 2007). "Computational Study on the Properties and Structure of Methyl Lactate". J. Phys. Chem. A. 111: 4671–4683. doi:10.1021/jp070841t.
- ↑ "Industrial Solvents Handbook" by Ernest W. Flick. 5th Edition. William Andrew Inc., 1998. ISBN 0-8155-1413-1, ISBN 978-0-8155-1413-8
