N-Acetyllactosamine
 | |
| Names | |
|---|---|
| IUPAC name
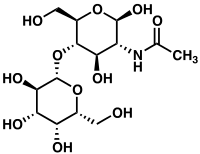
N-[(2R,3R,4R,5S,6R)-2,4-Dihydroxy-6-(hydroxymethyl)-5-[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-3-yl]acetamide | |
| Other names
β-D-galactopyranosyl-(1→4)-2-acetamido-2-deoxy-β-D-glucopyranose; 2-(Acetylamino)-2-deoxy-4-O-hexopyranosylhexopyranose | |
| Identifiers | |
| 32181-59-2 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:16153 |
| ChEMBL | ChEMBL457432 |
| ChemSpider | 7975931 |
| ECHA InfoCard | 100.164.310 |
| KEGG | C00611 |
| PubChem | 439271 |
| |
| Properties | |
| C14H25NO11 | |
| Molar mass | 383.35 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N-Acetyllactosamine also known as 2-(acetylamino)-2-deoxy-4-O-hexopyranosylhexopyranose is a nitrogen containing disaccharide.[1]
The N-acetyllactosamine is a component of many glycoproteins[2] and functions as a carbohydrate antigen that is thought to play roles in normal cellular recognition as well as in malignant transformation and metastasis.[3]
References
- ↑ Katzman RL (1972). "Isolation of N-Acetyllactosamine and Galactosyl-β-D-(1 → 4)-N-acetyllactosamine from Beef Brain Glycopeptides" (PDF). J. Biol. Chem. 247 (12): 3744–9. PMID 5033387.
- ↑ Zhou D (2003). "Why are glycoproteins modified by poly-N-acetyllactosamine glyco-conjugates?". Curr. Protein Pept. Sci. 4 (1): 1–9. PMID 12570780.
- ↑ Ito N, Yokota M, Nagaike C, Morimura Y, Hatake K, Matsunaga T (1996). "Histochemical demonstration and analysis of poly-N-acetyllactosamine structures in normal and malignant human tissues". Histol. Histopathol. 11 (1): 203–14. PMID 8720464.
External links
- N-acetyllactosamine at the US National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Wikipedia - version of the 8/4/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.