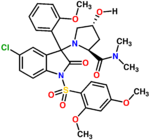
Nelivaptan
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | none |
| Identifiers | |
| |
| CAS Number |
439687-69-1 |
| PubChem (CID) | 9895468 |
| IUPHAR/BPS | 2202 |
| ChemSpider |
8071134 |
| UNII |
3TY57MQ4OA |
| ChEMBL |
CHEMBL582857 |
| ECHA InfoCard | 100.210.987 |
| Chemical and physical data | |
| Formula | C30H32ClN3O8S |
| Molar mass | 630.11 g/mol |
| |
| | |
Nelivaptan (INN[1]), codenamed SSR-149,415, is a selective and orally active non-peptide vasopressin receptor antagonist selective for the V1b subtype.[2] The drug had entered clinical trials for treatment of anxiety and depression.[3] In July 2008, Sanofi-Aventis announced that further development of this drug had been halted.[4]
References
- ↑ World Health Organization (2007). "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 98" (PDF). WHO Drug Information. 21 (4): 341.
- ↑ Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P (2002). "Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders". Proc. Natl. Acad. Sci. U.S.A. 99 (9): 6370–5. doi:10.1073/pnas.092012099. PMC 122955
 . PMID 11959912.
. PMID 11959912. - ↑ Serradeil-Le Gal C, Wagnon J, Tonnerre B, Roux R, Garcia G, Griebel G, Aulombard A (2005). "An overview of SSR149415, a selective nonpeptide vasopressin V(1b) receptor antagonist for the treatment of stress-related disorders". CNS Drug Reviews. 11 (1): 53–68. doi:10.1111/j.1527-3458.2005.tb00035.x. PMID 15867952.
- ↑ "Second-quarter 2008 results" (PDF). Press Release. Sanofi-Aventis. 2008-07-31. Retrieved 2009-06-10.
It has been decided to discontinue the development of amibegron and SSR 149415 (a V1B receptor antagonist).
External links
- SSR149415 at the US National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Wikipedia - version of the 6/6/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.