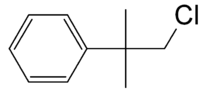
Neophyl chloride
 | |
| Names | |
|---|---|
| IUPAC name
(2-chloro-1,1-dimethylethyl)benzene | |
| Other names
(Chloro-tert-butyl)benzene | |
| Identifiers | |
| 515-40-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 61498 |
| ECHA InfoCard | 100.007.453 |
| PubChem | 68191 |
| |
| |
| Properties | |
| C10H13Cl | |
| Molar mass | 168.663 g/mol |
| Appearance | colorless liquid |
| Density | 1.047 g/cm3 |
| Boiling point | 223 °C (433 °F; 496 K) |
| organic solvents | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Neophyl chloride, C6H5C(CH3)2CH2Cl, is a halogenated organic compound with unusual nucleophilic substitution properties. Neophyl chloride is used to form a versatile organolithium reagent, neophyl lithium, by reaction with lithium.[1][2]
Preparation
Neophyl chloride was first synthesized by Haller and Ramart by reacting the chlorinating reagent thionyl chloride with neophyl alcohol:
- C6H5C(CH3)2CH2OH + SOCl2 → C6H5C(CH3)2CH2Cl + HCl + SO2
It is easily prepared on a large scale by combining benzene and methallyl chloride in the presence of a catalytic quantity of sulfuric acid.[3] The reaction is an example of an electrophilic aromatic substitution.
- H2C=C(CH3)CH2Cl + C6H6 → C6H5C(CH3)2CH2Cl
Alternatively tert-butylbenzene can be chlorinated with sulfuryl chloride.
Reactions and applications
Neophyl chloride can be used to form an organolithium reagent, neophyl lithium, by reaction with lithium. Organolithium reagents are useful due to their nucleophilic properties and their ability to form carbon-to-carbon bonds, like in reactions with carbonyls.
- C6H5C(CH3)2CH2Cl + Li → C6H5C(CH3)2CH2Li
Neophyl chloride is of interest to organic chemists due to its substitution properties. Neophyl chloride is a neopentyl halide which means it is subject to the neopentyl effect. This effect makes SN2 nucleophilic substitution highly unlikely because of steric interactions due to the branching of the β-carbon. No rotamer of the molecule would allow a backside attack of the α carbon.
β-Hydride elimination also does not occur with neophyl derivatives as this group lacks hydrogens at the β positions. These factors make neophyl chloride a precursor to intermediates that resist common substitution and elimination reactions.
References
- ↑ Lide, R. D.; CRC Handbook of Chemistry and Physics. 2003, 590. ISBN 0-8493-0595-0
- ↑ Andrew Streitwieser and Clayton H. Heathcock. Introduction to Organic Chemistry. 3rd ed. New York: Macmillan Co., 1985. ISBN 0-02-946720-9
- ↑ W. T. Smith, Jr. and J. T. Sellas (1963). "Neophyl chloride". Org. Synth.