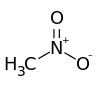
Nitromethane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Nitromethane[1] | |||
| Other names
Nitrocarbol | |||
| Identifiers | |||
| 75-52-5 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:77701 | ||
| ChEMBL | ChEMBL276924 | ||
| ChemSpider | 6135 | ||
| ECHA InfoCard | 100.000.797 | ||
| KEGG | C19275 | ||
| PubChem | 6375 | ||
| RTECS number | PA9800000 | ||
| |||
| |||
| Properties | |||
| CH3NO2 | |||
| Molar mass | 61.04 g/mol | ||
| Appearance | colorless, oily liquid[2] | ||
| Odor | Light, fruity[2] | ||
| Density | 1.1371 g/cm3 (20 °C) | ||
| Melting point | −28.38 °C (−19.08 °F; 244.77 K)[3] | ||
| Boiling point | 101.19 °C (214.14 °F; 374.34 K)[3] | ||
| ca. 10 g/100 mL | |||
| Solubility | miscible in diethyl ether, acetone, ethanol[4] | ||
| Vapor pressure | 28 mmHg (20°C)[2] | ||
| Acidity (pKa) | 17.2 in DMSO[5] | ||
| Refractive index (nD) |
1.3817 (20 °C) | ||
| Viscosity | 0.61 cP at 25 °C | ||
| Hazards | |||
| Main hazards | Flammable, harmful | ||
| Safety data sheet | See: data page | ||
| R-phrases | R5 R10 R22 | ||
| S-phrases | S41 | ||
| NFPA 704 | |||
| Flash point | 35 °C (95 °F; 308 K) | ||
| Explosive limits | 7.3%-?[2] | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) |
940 mg/kg (oral, rat) 950 mg/kg (oral, mouse)[6] | ||
| LDLo (lowest published) |
750 mg/kg (rabbit, oral) 125 mg/kg (dog, oral)[6] | ||
| LCLo (lowest published) |
7087 ppm (mouse, 2 hr) 1000 ppm (monkey) 2500 ppm (rabbit, 12 hr) 5000 ppm (rabbit, 6 hr)[6] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 100 ppm (250 mg/m3)[2] | ||
| REL (Recommended) |
none[2] | ||
| IDLH (Immediate danger) |
750 ppm[2] | ||
| Related compounds | |||
| Related nitro compounds |
nitroethane | ||
| Related compounds |
methyl nitrite methyl nitrate | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
| Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Nitromethane is an organic compound with the chemical formula CH
3NO
2. It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of pharmaceuticals, pesticides, explosives, fibers, and coatings.[7] Nitromethane is used as a fuel in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft.
Preparation
Nitromethane is produced industrially by treating propane with nitric acid at 350–450 °C (662–842 °F). This exothermic reaction produces the four industrially significant nitroalkanes: nitromethane, nitroethane, 1-nitropropane, and 2-nitropropane. The reaction involves free radicals, including the alkoxyl radicals of the type CH3CH2CH2O, which arise via homolysis of the corresponding nitrite ester. These alkoxy radicals are susceptible to C—C fragmentation reactions, which explains the formation of a mixture of products.[7]
Laboratory methods
It can be prepared in other methods that are of instructional value. The reaction of sodium chloroacetate with sodium nitrite in aqueous solution produces this compound:[8]
Uses
The principal use of nitromethane is as a stabilizer for chlorinated solvents, which are used in dry cleaning, semiconductor processing, and degreasing. It is also used most effectively as a solvent or dissolving agent for acrylate monomers, such as cyanoacrylates (more commonly known as "super-glue").[7]
Reactions
Acid-base properties
Nitromethane is a relatively acidic carbon acid. It has a has a pKa of 17.2 in DMSO solution. This value indicates an aqueous pKa of about 11.[9] It is slow to deprotonate. Protonation of the conjugate base O2NCH2-, which is nearly isosteric with nitrate, occurs initially at oxygen.[10]
Organic reactions
In organic synthesis nitromethane is employed as a one carbon building block.[11][12] Its acidity allows it to undergo deprotonation, enabling condensation reactions analogous to those of carbonyl compounds. Thus, under base catalysis, nitromethane adds to aldehydes in 1,2-addition in the nitroaldol reaction. Some important derivatives include the pesticides chloropicrin (Cl3CNO2), beta-nitrostyrene, and tris(hydroxymethyl)nitromethane, ((HOCH2)3CNO2). Reduction of the latter gives tris(hydroxymethyl)aminomethane, (HOCH2)3CNH2, better known as tris, a widely used buffer. In more specialized organic synthesis, nitromethane serves as a Michael donor, adding to α,β-unsaturated carbonyl compounds via 1,4-addition in the Michael reaction.
As an engine fuel
Nitromethane is used by hobbyists as a fuel in motor racing, particularly drag racing, as well as for radio-controlled models[7] (such as cars, planes and helicopters). In this context, nitromethane is commonly referred to as "nitro". The oxygen content of nitromethane enables it to burn with much less atmospheric oxygen.
- 4 CH3NO2 + 3 O2 → 4 CO2 + 6 H2O + 2 N2
The amount of air required to burn 1 kg (2.2 lb) of gasoline is 14.7 kg (32 lb), but only 1.7 kg (3.7 lb) of air is required for 1 kg of nitromethane. Since an engine's cylinder can only contain a limited amount of air on each stroke, 8.7 times more nitromethane than gasoline can be burned in one stroke. Nitromethane, however, has a lower specific energy: gasoline provides about 42–44 MJ/kg, whereas nitromethane provides only 11.3 MJ/kg. This analysis indicates that nitromethane generates about 2.3 times the power of gasoline when combined with a given amount of oxygen.
Nitromethane can also be used as a monopropellant, i.e., a fuel that burns without added oxygen. The following equation describes this process:
- 2 CH3NO2 → 2 CO + 2 H2O + H2 + N2
Nitromethane has a laminar combustion velocity of approximately 0.5 m/s, somewhat higher than gasoline, thus making it suitable for high-speed engines. It also has a somewhat higher flame temperature of about 2,400 °C (4,350 °F). The high heat of vaporization of 0.56 MJ/kg together with the high fuel flow provides significant cooling of the incoming charge (about twice that of methanol), resulting in reasonably low temperatures
Nitromethane is usually used with rich air–fuel mixtures because it provides power even in the absence of atmospheric oxygen. When rich air–fuel mixtures are used, hydrogen and carbon monoxide are two of the combustion products. These gases often ignite, sometimes spectacularly, as the normally very rich mixtures of the still burning fuel exits the exhaust ports. Very rich mixtures are necessary to reduce the temperature of combustion chamber hot parts in order to control pre-ignition and subsequent detonation. Operational details depend on the particular mixture and engine characteristics.
A small amount of hydrazine blended in nitromethane can increase the power output even further. With nitromethane, hydrazine forms an explosive salt that is again a monopropellant. This unstable mixture poses a severe safety hazard and is forbidden for use in the United States[13] for model aircraft fuels, which has also banned tetranitromethane for similar reasons of volatility.
In model aircraft and car glow fuel, the primary ingredient is generally methanol with some nitromethane (0% to 65%, but rarely over 30%, and 10–20% lubricants (usually castor oil and/or synthetic oil). Even moderate amounts of nitromethane tend to increase the power created by the engine (as the limiting factor is often the air intake), making the engine easier to tune (adjust for the proper air/fuel ratio).
Explosive properties
Nitromethane was not known to be a high explosive until a railroad tanker car loaded with it exploded on June 1, 1958.[14] After much testing, it was realized that nitromethane was a more energetic high explosive than TNT, although TNT has a higher velocity of detonation (VoD) and brisance. Both of these explosives are oxygen-poor, and some benefits are gained from mixing with an oxidizer, such as ammonium nitrate. Pure nitromethane is an insensitive explosive with a VoD of approximately 6,400 m/s (21,000 ft/s), but even so inhibitors may be used to reduce the hazards. The tank car explosion was speculated to be due to adiabatic compression, a hazard common to all liquid explosives. This is when small entrained air bubbles compress and superheat with rapid rises in pressure. It was thought that an operator rapidly snapped shut a valve creating a "hammer-lock" pressure surge.
Nitromethane can also be mixed with ammonium nitrate, which is used as an oxidizer, to form an explosive mixture known as ANNM. One graphic example of this was the use of nitromethane and ammonium nitrate in the Oklahoma City bombing.
Nitromethane exhaust
Exhaust gas from an internal combustion engine whose fuel includes nitromethane will contain nitric acid vapour, which is corrosive, and when inhaled causes a muscular reaction making it impossible to breathe. People exposed to it should wear a gas mask.[15] The condensed nitric acid-based residue left over in a glow-fueled model engine after a model-flight session can also corrode their internal components, usually mandating use of a combination of kerosene to neutralize the residual nitric acid, and an "after-run oil" (often the lower-viscosity "air tool oil" variety of a popular preservative oil) for lubrication to safeguard against such damage, when such an engine is placed into storage.
Purification
Nitromethane is a popular solvent in organic and electroanalytical chemistry. It can be purified by cooling below its freezing point, washing the solid with cold diethyl ether, followed by distillation.[16]
See also
- Top Fuel
- Adiabatic flame temperature, a thermodynamic calculation of the flame temperature of nitromethane
- Dinitromethane
- Trinitromethane
- Tetranitromethane
- RE factor
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 662. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- 1 2 3 4 5 6 7 "NIOSH Pocket Guide to Chemical Hazards #0457". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 David R. Lide (ed), CRC Handbook of Chemistry and Physics, 84th Edition. CRC Press. Boca Raton, Florida, 2003; Section 3, Physical Constants of Organic Compounds
- ↑ http://chemister.ru/Database/properties-en.php?dbid=1&id=27
- ↑ Reich, Hans. "Bordwell pKa table: "Nitroalkanes"". University of Wisconsin Chemistry Department. Retrieved 17 January 2016.
- 1 2 3 "Nitromethane". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 4 Markofsky, S. B. (2000). "Nitro Compounds, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_401.pub2.
- ↑ Whitmore, F. C.; Whitmore, M. G. (1941). "Nitromethane". Org. Synth.; Coll. Vol., 1, p. 401
- ↑ Bordwell, F. G.; Satish, A. V., "Is Resonance Important in Determining the Acidities of Weak Acids or the Homolytic Bond Dissociation Enthalpies (BDEs) of Their Acidic H-A Bonds?", J. Am. Chem. Soc. 1994, volume 116, 8885-8889. doi:10.1021/ja00099a004
- ↑ Kramarz, K. W.; Norton, J. R., "Slow Proton Transfer Reactions in Organometallic and Bioinorganic Chemistry", Prog. Inorg. Chem. 1994, 42, 1-65.
- ↑ Dauben, H. J. Jr.; Ringold, H. J.; Wade, R. H.; Pearson, D. L.; Anderson, A. G. Jr.; de Boer, T. J.; Backer, H. J. (1963). "Cycloheptanone". Org. Synth.; Coll. Vol., 4, p. 221
- ↑ Noland, W. E. (1963). "2-Nitroethanol". Org. Synth.; Coll. Vol., 4, p. 833
- ↑ "AMA Competition Regulations 2015-2016 Part 7. Fuels" (PDF). www.modelaircraft.org. Academy of Model Aeronautics. February 15, 2016. p. 24. Retrieved April 18, 2014.
- ↑ Interstate Commerce Commission. "Accident Near Mt. Pulaski, ILL" (PDF). Ex Parte No 213.
- ↑ turbofast.com
- ↑ Coetzee, J. F.; Chang, T.-H. (1986). "Recommended Methods for the Purification of Solvents and Tests for Impurities: Nitromethane" (PDF). Pure and Applied Chemistry. 58 (11): 1541–1545. doi:10.1351/pac198658111541.