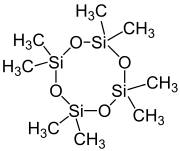
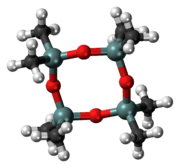
Octamethylcyclotetrasiloxane
 | |
 | |
| Names | |
|---|---|
| Other names
D4, OMCTS | |
| Identifiers | |
| 556-67-2 | |
| ECHA InfoCard | 100.008.307 |
| Properties | |
| C8H24O4Si4 | |
| Molar mass | 296.62 |
| Density | 0.956 g/mL |
| Melting point | 17–18 °C (63–64 °F; 290–291 K) |
| Boiling point | 175–176 °C (347–349 °F; 448–449 K) |
| 56.2±2.5 ppb (23 °C) [1] | |
| Vapor pressure | 124.5±6.2 Pa (25 °C) [2] |
| Related compounds | |
| Related compounds |
Disiloxane Tetramethylsilane |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Octamethylcyclotetrasiloxane, also called D4, is an organosilicon compound with the formula [(CH3)2SiO]4. lt is a colorless viscous liquid. It is a common cyclomethicone. Like other cyclomethicones, it is slightly volatile. It has attracted scrutiny because it is pervasive in the environment.[3]
Production
Commercially, D4 is produced by cracking polysiloxanes. The silicone polymer equilibrates in the presence of a strong base to give the tetramer
- n/4 [(CH3)2SiO]n → n [(CH3)2SiO]4
The pentamer decamethylcyclopentasiloxane is also generated. These two cyclic species are separated from the polymer by distillation.[4]
Occurrence
It is among the most important of all the cyclic siloxanes, with a global production volume of 136·106 kilograms in 1993.[5]
As the smallest stable cyclic siloxane, D4 is one of the most abundant siloxanes in the environment, e.g. in landfill gases.[6]
Environment
D5 and D4 have attracted attention because they are pervasive. Although never acutely toxic, one report suggests cyclic siloxanes can be detected in some species of aquatic life.[3] However, other scientific reviews have determined that "Siloxane D5 does not pose a danger to the environment."[7]
References
- ↑ Sudarsanan Varaprath; Cecil L. Frye; Jerry Hamelink (1996). "Aqueous solubility of permethylsiloxanes (silicones)". Environmental Toxicology and Chemistry. 15 (8): 1263–1265. doi:10.1002/etc.5620150803.
- ↑ Ying Duan Lei; Frank Wania; Dan Mathers (2010). "Temperature-Dependent Vapor Pressure of Selected Cyclic and Linear Polydimethylsiloxane Oligomers". Journal of Chemical & Engineering Data. 55 (12): 5868–5873. doi:10.1021/je100835n.
- 1 2 Wang, De-Gao; Norwood, Warren; Alaee, Mehran; Byer, Jonathan D.; Brimble, Samantha "Review of Recent Advances in Research on the Toxicity, Detection, Occurrence and Fate of Cyclic Volatile Methyl Siloxanes in the Environment" Chemosphere 2013, volume 93, pages 711–725. doi:10.1016/j.chemosphere.2012.10.041
- ↑ Moretto, Hans-Heinrich; Schulze, Manfred; Wagner, Gebhard (2005). "Silicones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_057.
- ↑ Philippe P. Quevauviller; Patrick Roose; Gert Verreet (24 August 2011). Chemical Marine Monitoring: Policy Framework and Analytical Trends. John Wiley & Sons. p. 2010. ISBN 978-1-119-97759-9.
- ↑ Franz-Bernd Frechen (2009). Odours and VOCs: Measurement, Regulation and Control Techniques. kassel university press GmbH. p. 287. ISBN 978-3-89958-609-1.
- ↑ Report of the Board of Review for Decamethylcyclopentasiloxane (Siloxane D5) established under Section 333(1) of the Canadian Environmental Protection Act of 1999, October 20, 2011