Orellanine
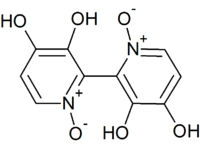 | |
| Names | |
|---|---|
| IUPAC name
3,3′,4,4′-Tetrahydroxy-2,2′-bipyridine-N,N′-dioxide | |
| Other names
Orellanin, 2,2-Bipyridine-3,3-4,4-tetrol-1,1-dioxide | |
| Identifiers | |
| 37338-80-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 10266115 (N-oxide form) 80848 (non-zwitterionic form) |
| ECHA InfoCard | 100.232.424 |
| |
| |
| Properties | |
| C10H8N2O6 | |
| Molar mass | 252.18 g·mol−1 |
| Hazards | |
| Main hazards | Highly toxic |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Orellanine or orellanin is a mycotoxin found in a group of mushrooms known as the Orellani of the Cortinariaceae family.[1] Structurally, it is a pyridine N-oxide.
History
In Poland during the 1950s there was a small epidemic where over 100 people became ill. What caused the illness remained a mystery until 1952 when Polish physician Dr. Stanisław Grzymala[2] discovered that everyone suffering from the illness, which had by now claimed several lives, had eaten the mushroom Cortinarius orellanus.[3]
In 1962 he isolated a substance from the fungus. He named it orellanine after the Latin name of the toadstool. Given orally to research animals, he produced the same reaction as in humans.[4]
In 1973 orellanine was discovered in the toadstool Cortinarius rubellus.
Chemistry
The chemical constitution of orellanine remained unknown until the Polish chemists Antkowiak and Gessner in the last half of the 1970s discovered that it was a bipyridine dioxide.[5][6] Orellanine undergoes tautomerization, and the more stable tautomer is the amine oxide form.

An interesting feature of orellanine is its ability to bind aluminium ions to form chelation complexes.[7]
Toxicity
Bipyridines with positively charged nitrogen atoms were already known to be poisonous before the structure of orellanine was elucidated. The herbicides paraquat and diquat are toxic not only to plants, but also to humans and livestock. Bipyridines with charged nitrogen atoms disrupt important redox reactions in organisms, ‘stealing’ one or two electrons and sometimes bypass the electrons into other and often undesirable redox reactions. The terminal product can be peroxide or superoxide ions, the latter of which is harmful to the cells. It is likely that orellanine works in the same way, although the process from disturbed redox reactions to the serious clinical kidney damage has not been properly resolved.
In humans, a characteristic of poisoning by the nephrotoxin orellanine is the long latency; the first symptoms usually do not appear until 2–3 days after ingestion and can in some cases take as long as 3 weeks. The first symptoms of orellanine poisoning are similar to the common flu (nausea, vomiting, stomach pains, headaches, myalgia, etc.), these symptoms are followed by early stages of renal failure (immense thirst, frequent urination, pain on and around the kidneys) and eventually decreased or nonexistent urine output and other symptoms of renal failure occur. If left untreated death will follow.
The LD50 of orellanine in mice is 12 to 20 mg per kg body weight;[8][9] this is the dose which leads to death within two weeks. From cases of orellanine-related mushroom poisoning in humans it seems that the lethal dose for humans is considerably lower.
Treatment
Although there is no known antidote against orellanine poisoning, early hospitalization can sometimes prevent serious injury and usually prevent death. Research is ongoing. Some treatments make use of anti-oxidant therapy and corticosteroids to help victims recover from their renal failure.[10]
See also
References
- ↑ Oubrahim H.; Richard J.-M.; Cantin-Esnault D.; Seigle-Murandi F.; Trecourt F (1997). "Novel methods for identification and quantification of the mushroom nephrotoxin orellanine". Journal of Chromatography. 758 (1): 145–157. doi:10.1016/S0021-9673(96)00695-4. PMID 9181972.
- ↑ See:
- Alina Skirgiełło and Andrzej Nespiak (1957) "Erfahrungen mit Dermocybe orellana (Fr.) in Polen: A. Cortinarius (Dermocybe) orellanus Fr. non Quél. — Cause d'intoxications fongiques en Pologne en 1952-55" (Experiences with Dermocybe orellana (Fr.) in Poland: A. Cortinarius (Dermocybe) orellanus Fr. non Quél. — Cause of mushroom poisoning in Poland in 1952-55) Zeitschrift für Pilzkunde (Journal for Mycology), vol. 23, pages 138-139 ;
- Stanisław Grzymala (1957) "Erfahrungen mit Dermocybe orellana (Fr.) in Polen: B. Massenvergiftung durch den Orangefuchsigen Hautkopf. (Experiences with Dermocybe orellana (Fr.) in Poland: B. Mass poisoning by the orange-red web-cap), Zeitschrift für Pilzkunde, vol. 23, pages 139-142 ;
- Grzymala, S. (1959) "Zur toxischen Wirkung des orangefuchsigen Hautkopfes (Dermocybe orellana Fr.)" (On the toxic effect of the orange-red web-cap (Dermocybe orellana Fr.)), Deutsche Zeitschrift für die gesamte gerichtliche Medizin (German Journal for Forensic Medicine), vol. 49, pages 91-99 ;
- Grzymala, S. (1962) "L'isolement de l'orellanine poison du Cortinarius orellanus Fries et l'étude de ses effects anatomopathologiques" (Isolation of orellanine, poison of Cortinarius orellanus Fries, and study of its histopathological effects), Bulletin de la Société Mycologique de France, vol. 78, pages 394-404.
- ↑ Spoerke, David G.; Barry H Rumack (1994). Handbook of Mushroom Poisoning: Diagnosis and Treatment. CRC Press. p. 250. ISBN 0-8493-0194-7.
- ↑ Slørsoppene omfatter noen av våre farligste giftsopper [Veiled mushrooms include some of our most dangerous toxic mushrooms] (Article in Norwegian)
- ↑ Antkowiak W.Z., Gessner W. P. (1975). "Isolation and characteristics of toxic components of Cortinarius orellanus, Fries". Bulletin de l'Academie Polonaise des Sciences, Serie des Sciences Chemiques. 23: 729–733.
- ↑ Antkowiak Wieław Z., Gessner Wiesł P. (1979). "The structures of orellanine and orelline". Tetrahedron Letters. 20 (21): 1931–1934. doi:10.1016/s0040-4039(01)86882-9.
- ↑ Høiland K (1994). "Suppression of the toxic effect of soluble aluminium on fungi by dermocybin-1-ß-D-glucopyranoside and orellanine from Cortinarius sanguineus and C. orellanoides". Nord. J. Bot. 14: 221–228. doi:10.1111/j.1756-1051.1994.tb00591.x.
- ↑ Prast H; Werner ER; Pfaller W; Moser M. (1988). "Toxic properties of the mushroom Cortinarius orellanus. I. Chemical characterization of the main toxin of Cortinarius orellanus (Fries) and Cortinarius speciosissimus (Kuhn & Romagn) and acute toxicity in mice". Archives of Toxicology. 62 (1): 81–8. doi:10.1007/bf00316263. PMID 3190463.
- ↑ Holmdahl, J (2001). Mushroom poisoning: Cortinarius speciosissimus nephrotoxicity (PDF). Göteborg University. Archived February 4, 2012, at the Wayback Machine.
- ↑ Rachael G. Kilner; et al. (1999). "Acute renal failure from intoxication by Cortinarius orellanus: recovery using anti-oxidant therapy and steroids". Nephrology Dialysis Transplantation. 14 (11): 2779–2780. doi:10.1093/ndt/14.11.2779-a.
External links
- Cortinarius rubellus Pacific Northwest Fungi, Featured Fungus Number 4