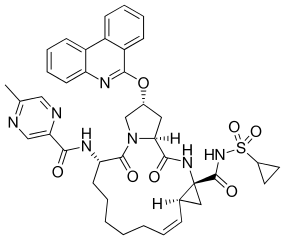
Paritaprevir
 | |
| Clinical data | |
|---|---|
| Trade names | Viekira Pak (in combination with ombitasvir, ritonavir and dasabuvir), Technivie/Viekirax (in combination with ombitasvir and ritonavir) |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | J05AX66 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | was not evaluated |
| Protein binding | 97–98.6% |
| Metabolism | hepatic, CYP3A4 and CYP3A5 |
| Onset of action | 4 to 5 hours |
| Biological half-life | 5.5 hours |
| Excretion | feces (88%), urine (8,8%) |
| Identifiers | |
| |
| Synonyms | Veruprevir; ABT-450 |
| CAS Number | 1216941-48-8 |
| PubChem (CID) | 45110509 |
| ChemSpider | 32700634 |
| KEGG | D10580 |
| ChEBI |
CHEBI:85188 |
| ChEMBL | CHEMBL3391662 |
| Chemical and physical data | |
| Formula | C40H43N7O7S |
| Molar mass | 765.88 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Paritaprevir (previously known as ABT-450) is an acylsulfonamide[1] inhibitor of the NS3-4A serine protease[2] manufactured by Abbott Laboratories[3] that shows promising results as a treatment of hepatitis C. When given in combination with ritonavir and ribavirin for 12 weeks, the rate of sustained virologic response at 24 weeks after treatment has been estimated to be 95% for those with hepatitis C virus genotype 1.[4] Resistance to treatment with paritaprevir is uncommon, because it targets the binding site, but has been seen to arise due to mutations at positions 155 and 168 in NS3.[5]p. 248
Paritaprevir is a component of Viekira Pak and Technivie.[6]
References
- ↑ Seng-Lai Tan; Yupeng He, eds. (2011). Hepatitis C: antiviral drug discovery and development. Norfolk: Caister academic press. p. 210. ISBN 9781904455783. Retrieved 28 April 2014.
- ↑ Donald Jensen; Nancy Reau, eds. (2013). Hepatitis C. New York: Oxford University Press. p. 144. ISBN 9780199844296. Retrieved 28 April 2014.
- ↑ "Abbott Announces Phase 3 Hepatitis C Program Details". Abbott company website. Abbott Laboratories. Retrieved 28 April 2014.
- ↑ Kowdley, Kris V.; Lawitz, Eric; Poordad, Fred; Cohen, Daniel E.; Nelson, David R.; Zeuzem, Stefan; Everson, Gregory T.; Kwo, Paul; Foster, Graham R.; Sulkowski, Mark S.; Xie, Wangang; Pilot-Matias, Tami; Liossis, George; Larsen, Lois; Khatri, Amit; Podsadecki, Thomas; Bernstein, Barry (2014). "Phase 2b Trial of Interferon-free Therapy for Hepatitis C Virus Genotype 1". New England Journal of Medicine. 370 (3): 222–232. doi:10.1056/NEJMoa1306227. ISSN 0028-4793.
- ↑ Stefan Mauss; et al., eds. (2013). Hepatology 2013 a clinical textbook (PDF) (4th ed.). Düsseldorf: Flying Publisher. ISBN 978-3-924774-90-5. Retrieved 28 April 2014.
- ↑ "TECHNIVIE™ (ombitasvir, paritaprevir and ritonavir) Tablets, for Oral Use. Full Prescribing Information" (PDF). AbbVie Inc., North Chicago, IL 60064. Retrieved 28 July 2015.
This article is issued from Wikipedia - version of the 9/21/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.