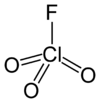
Perchloryl fluoride
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Perchloryl fluoride | |||
| Other names
Chlorine oxyfluoride, Perchlorofluoride, Chlorine fluorine oxide, Trioxychlorofluoride, Perchloric acid fluoride | |||
| Identifiers | |||
| 7616-94-6 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 22680 | ||
| ECHA InfoCard | 100.028.660 | ||
| EC Number | 231-526-0 | ||
| PubChem | 24258 | ||
| RTECS number | SD1925000 | ||
| |||
| |||
| Properties | |||
| ClO3F | |||
| Molar mass | 102.4496 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | sweet odor | ||
| Density | 1.434 g/cm3 | ||
| Melting point | −147.8 °C (−234.0 °F; 125.3 K) | ||
| Boiling point | −46.7 °C (−52.1 °F; 226.5 K) | ||
| 0.06 g/100 ml (20 °C) | |||
| Vapor pressure | 10.5 atm (20°C)[1] | ||
| Viscosity | 3.91 x 10−3 Pa.s (@ melting point) | ||
| Structure | |||
| Tetrahedral[2]:373 | |||
| Thermochemistry | |||
| Std enthalpy of formation (ΔfH |
−5.7[2]:380 | ||
| Hazards | |||
| Main hazards | Corrosive, oxidizing, toxic | ||
| NFPA 704 | |||
| 3 ppm | |||
| Lethal dose or concentration (LD, LC): | |||
| LC50 (median concentration) |
385 ppm (rat, 4 hr) 451 ppm (dog, 4 hr)[3] | ||
| LCLo (lowest published) |
2000 ppm (rat, 40 min) 451 ppm (dog, 4 hr)[3] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 3 ppm (13.5 mg/m3)[1] | ||
| REL (Recommended) |
TWA 3 ppm (14 mg/m3) ST 6 ppm (28 mg/m3)[1] | ||
| IDLH (Immediate danger) |
100 ppm[1] | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Perchloryl fluoride[4] is a reactive gas with the chemical formula ClO
3F. It has a characteristic sweet odor[5] that resembles gasoline and kerosene. It is toxic and is a powerful oxidizing and fluorinating agent. It is the acid fluoride of perchloric acid.
In spite of its small enthalpy of formation (ΔHfO = −5.7), it is stable, decomposing only at 400 °C.[2]:380 It is quite reactive towards reducing agents and anions, however, with the chlorine atom acting as an electrophile.[2]:382 It reacts explosively with reducing agents such as amides, metals, hydrides, etc.[5] Its hydrolysis in water occurs very slowly, unlike that of chloryl fluoride.
Synthesis and chemistry
Perchloryl fluoride is produced primarily by the fluorination of perchlorates. Antimony pentafluoride is a commonly used fluorinating agent:[2]:372–373
- ClO−
4 + 3 HF + 2 SbF
5 → ClO
3F + H
3O+
+ 2 SbF−
6
ClO
3F reacts with alcohols to produce alkyl perchlorates, which are extremely shock-sensitive explosives.[6] Using Friedel-Crafts catalysts, it can be used for introducing the –ClO
3 group into aromatic rings via electrophilic aromatic substitution.[7]
Applications
Perchloryl fluoride is used in organic chemistry as a mild fluorinating agent.[2]:383 It was the first industrially relevant electrophilic fluorinating agent, used since the 1960s for producing fluorinated steroids.[6] In the presence of aluminum trichloride, it has also been used as a electrophilic perchlorylation reagent for aromatic compounds.[8]
Perchloryl fluoride was investigated as a high performance liquid rocket fuel oxidizer.[9] In comparison with chlorine pentafluoride and bromine pentafluoride, it has significantly lower specific impulse, but does not tend to corrode tanks. It does not require cryogenic storage.
It can also be used in flame photometry as an excitation source.[10]
Safety
Perchloryl fluoride is toxic, with a TLV of 3 ppm.[11] It is a strong lung- and eye-irritant capable of producing burns on exposed skin. Its IDLH level is 100 ppm.[12] Symptoms of exposure include dizziness, headaches, syncope, and cyanosis. Exposure to toxic levels causes severe respiratory tract inflammation and pulmonary edema.[9]
References
- 1 2 3 4 "NIOSH Pocket Guide to Chemical Hazards #0490". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 4 5 6 Harry Julius Emeléus; A. G. Sharpe (1976). Advances in inorganic chemistry and radiochemistry, Volume 18. Academic Press. ISBN 0-12-023618-4.
- 1 2 "Perchloryl fluoride". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ Chemical Science and Technology Laboratory. "Perchloryl fluoride". National Institute of Standards and Technology. Retrieved 2009-11-28.
- 1 2 Jared Ledgard (2007). The Preparatory Manual of Explosives (3rd ed.). Lulu.com. p. 77. ISBN 0-615-14290-7.
- 1 2 Peer Kirsch (2004). Modern fluoroorganic chemistry: synthesis, reactivity, applications. Wiley-VCH. p. 74. ISBN 3-527-30691-9.
- ↑ Peter Bernard David De la Mare (1976). Electrophilic halogenation: reaction pathways involving attack by electrophilic halogens on unsaturated compounds. CUP Archive. p. 63. ISBN 0-521-29014-7.
- ↑ Inman, C. E.; Oesterling, R. E.; Tyczkowski, E. A. (1958-10-01). "Reactions of Perchloryl Fluoride with Organic Compounds. I. Perchlorylation of Aromatic Compounds1". Journal of the American Chemical Society. 80 (19): 5286–5288. doi:10.1021/ja01552a069. ISSN 0002-7863.
- 1 2 John Burke Sullivan; Gary R. Krieger (2001). Clinical environmental health and toxic exposures (2nd ed.). Lippincott Williams & Wilkins. p. 969. ISBN 0-683-08027-X.
- ↑ Schmauch, G. E.; Serfass, E. J. (1958). "The Use of Perchloryl Fluoride in Flame Photometry". Applied Spectroscopy. 12 (3): 98–102. Bibcode:1958ApSpe..12...98S. doi:10.1366/000370258774615483.
- ↑ National Institute for Occupational Safety and Health. "NIOSH Pocket Guide to Chemical Hazards". Centers for Disease Control and Prevention. Retrieved 2013-10-31.
- ↑ National Institute for Occupational Safety and Health. "Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs)". Centers for Disease Control and Prevention. Retrieved 2013-10-31.