Pinacol
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dimethylbutane-2,3-diol | |
| Other names
2,3-Dimethyl-2,3-butanediol Tetramethylethylene glycol 1,1,2,2-Tetramethylethylene glycol Pinacone | |
| Identifiers | |
| 76-09-5 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:131185 |
| ChemSpider | 21109330 |
| ECHA InfoCard | 100.000.849 |
| |
| |
| Properties | |
| C6H14O2 | |
| Molar mass | 118.174 g/mol |
| Appearance | White solid |
| Density | 0.967 g/cm3 |
| Melting point | 40 to 43 °C (104 to 109 °F; 313 to 316 K) |
| Boiling point | 171 to 173 °C (340 to 343 °F; 444 to 446 K) |
| Hazards | |
| Safety data sheet | External MSDS |
| S-phrases | S24 S25 |
| Flash point | 77 °C (171 °F; 350 K) |
| Related compounds | |
| Related compounds |
Pinacolone |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Pinacol is a white solid organic compound. It is a diol that has hydroxyl groups (-OH) on vicinal carbon atoms.
Preparation
It may be produced by the pinacol coupling reaction from acetone:[1]
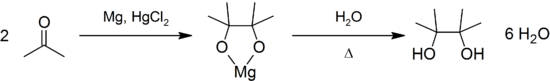
Reactions
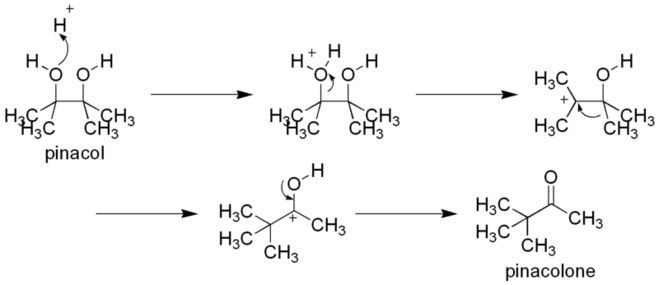
As a vicinal-diol, it can rearrange to pinacolone by the pinacol rearrangement, e.g. by heating with sulfuric acid:[2]
Pinacol can be used with borane and boron trichloride to produce useful synthetic intermediates such as pinacolborane, bis(pinacolato)diboron,[3] and pinacolchloroborane.
See also
References
- ↑ Roger Adams and E. W. Adams. "Pinacol Hydrate". Org. Synth.; Coll. Vol., 1, p. 459
- ↑ G. A. Hill and E. W. Flosdorf (1941). "Pinacolone". Org. Synth.; Coll. Vol., 1, p. 462
- ↑ Tatsuo Ishiyama, Miki Murata, Taka-aki Ahiko, and Norio Miyaura (2004). "Bis(pinacolato)diboron". Org. Synth.; Coll. Vol., 10, p. 115
This article is issued from Wikipedia - version of the 9/11/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.