Pinocembrin
 | |
| Names | |
|---|---|
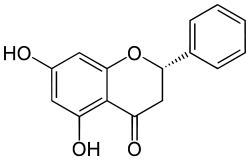
| IUPAC name
(2S)-5,7-dihydroxy-2-phenyl-2,3-dihydro-4H-chromen-4-one | |
| Other names
Dihydrochrysin Galangin flavanone 5,7-Dihydroxyflavanone 5,7-dihydroxy-2-phenyl-2,3-dihydro-4H-chromen-4-one 4H-1-benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-phenyl- 5,7-Dihydroxy-2-phenyl-chroman-4-one | |
| Identifiers | |
| 480-39-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:28157 |
| ChEMBL | ChEMBL399910 |
| ChemSpider | 208593 |
| PubChem | 238782 |
| |
| |
| Properties | |
| C15H12O4 | |
| Molar mass | 256.25 g/mol |
| Density | 1.386 g/mL |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Pinocembrin is a flavanone, a type of flavonoid. It is an antioxidant found in damiana,[1] honey, fingerroot,[2] and propolis.[3]
Pinocembrin can be converted biosynthetically to pinobanksin by hydroxylation adjacent to the ketone. Studies have shown that pinocembrin has potential as a drug to treat cerebral ischemia, neurodegenerative diseases, cardiovascular diseases and atherosclerosis as well as other clinical conditions.[4]
See also
References
- ↑ Zhao, J; Dasmahapatra AK; Khan SI; Khan IA (Dec 2008). "Anti-aromatase activity of the constituents from damiana (Turnera diffusa)". J Ethnopharmacol. 120 (3): 387–393. doi:10.1016/j.jep.2008.09.016. PMID 18948180.
- ↑ Punvittayagul, C; Wongpoomchai R; Taya S; Pompimon W. (January 2011). "Effect of pinocembrin isolated from Boesenbergia pandurata on xenobiotic-metabolizing enzymes in rat liver.". Drug Metabolism Letters. 5 (1): 1–5. doi:10.2174/187231211794455226. PMID 20942797.
- ↑ Bosio K; Avanzini C; D’Avolio A; Ozino O; Savoia D (2000). "In vitro activity of propolis against Streptococcus pyogenes". Letters in Applied Microbiology. 31 (2): 174–177. doi:10.1046/j.1365-2672.2000.00785.x. PMID 10972723.
- ↑ Lan X, Wang W, Li Q, Wang J (April 2016). "The natural flavonoid pinocembrin: molecular targets and potential therapeutic applications". Mol Neurobiol. 53 (3): 1794–801. doi:10.1007/s12035-015-9125-2. PMC 4561606
 . PMID 25744566.
. PMID 25744566.
This article is issued from Wikipedia - version of the 11/8/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.