Plitidepsin
 | |
| Names | |
|---|---|
| IUPAC name
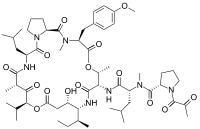
(S)-N-((R)-1-(((3S,6R,7S,10R,11S,15S,17S,20S,25aS)-10-((S)-sec-butyl)-11-hydroxy-20-isobutyl-15-isopropyl-3-(4-methoxybenzyl)-2,6,17-trimethyl-1,4,8,13,16,18,21-heptaoxodocosahydro-1H-pyrrolo[2,1-f][1,15,4,7,10,20]dioxatetraazacyclotricosin-7-yl)amino)-4-methyl-1-oxopentan-2-yl)-N-methyl-1-(2-oxopropanoyl)pyrrolidine-2-carboxamide | |
| Other names
Aplidine; Dehydrodidemnin B; Aplidin; N-[1-(1,2-Dioxopropyl)-L-prolyl]didemnin A | |
| Identifiers | |
| 137219-37-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:90205 |
| ChemSpider | 26001665 |
| |
| |
| Properties | |
| C57H87N7O15 | |
| Molar mass | 1,110.36 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Plitidepsin (also known as dehydrodidemnin B, marketed by PharmaMar, S.A. under the trade name Aplidin) is a chemical compound extracted from the ascidian Aplidium albicans.[1] It is currently undergoing clinical trial testing. It is a member of the class of compounds known as didemnins.
Chemical structure
Plitidepsin is a cyclic depsipeptide, meaning it is a cyclic peptide in which there is one or more ester bond in place of one or more of a peptide bond. Its chemical structure is very close to that of didemnin B, the only difference being that the lactate residue in didemnin B is present in the oxidized pyruvate version.
Pharmacological activity
Like all didemnin compounds, plitidepsin exhibits antitumor, antiviral and immunosuppressive activities. It shows promise in shrinking tumors in pancreatic, stomach, bladder, and prostate cancers.[2][3]
Clinical trials
In July 2003, plitidepsin was granted orphan drug status by the European Medicines Agency for treating acute lymphoblastic leukemia.[4]
As of 2007, it was undergoing multicenter phase II clinical trials.[3]
In 2016, early results in a small phase I trial for multiple myeloma were announced.[5]
References
- ↑ Cragg, G.M. & Newman, D.J. (2004). "Marine natural products and related compounds in clinical and advanced preclinical trials". Journal of Natural Products. 67 (8): 1216–1238. doi:10.1021/np040031y. PMID 15332835.
- ↑ Garrison, Tom. Oceanography: An Invitation to Marine Science 4th ed. United States: Brooks/Cole. 2002. 98.
- 1 2 Adrio, J.; et al. (2007). "Total Synthesis and Biological Evaluation of Tamandarin B Analogues". Journal of Organic Chemistry. 72 (14): 5129–5138. doi:10.1021/jo070412r.
- ↑ Public Summary of Positive Opinion for Orphan Designation of Aplidine for the Treatment of Acute Lymphoblastic Leukaemia
- ↑ ASCO: Plitidepsin Shows Activity in Multiple Myeloma. June 2016