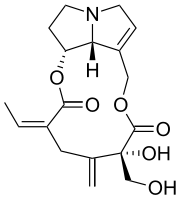
Riddelliine
 | |
| Names | |
|---|---|
| IUPAC name
(15E)-12,18-Dihydroxy-13,19-didehydrosenecionan-11,16-dione | |
| Other names
Riddelline; Riddelliine; Riddeline; Riddelliin | |
| Identifiers | |
| 23246-96-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 17215841 |
| PubChem | 5281744 |
| |
| |
| Properties | |
| C18H23NO6 | |
| Molar mass | 349.38 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Riddelliine (not to be confused with Ritalin) is a chemical compound classified as a pyrrolizidine alkaloid. It was first isolated from Senecio riddellii[1] and is also found in a variety of plants including Jacobaea vulgaris, Senecio vulgaris, and others plants in the genus Senecio.
Riddelliine can be found as a contaminant in foods such as meat, grains, seeds, milk, herbal tea, and honey.[2]
Riddelliine is suspected to be a carcinogen.[3] It is listed as an IARC Group 2B carcinogen and listed by the National Toxicology Program in its Report on Carcinogens which lists chemicals "known or reasonably anticipated to cause cancer in humans".[4]
History
The first reported isolation of ‘Riddelliine’ was done by Richard H. F. Manske, a chemist at the National Research Laboratories in Ottawa, Canada. This was handed in on the 13th dezember 1938 and published in the 17th volume, section B in the Canadian Journal of Research, January 1939.[5]
The formula was confirmed and a structure was added by Roger Adams, K.E. Hamlin, JR., C.F. Jelinek and R.F. Phillips in the Journal of the American Chemical Society 1942.[6]
Structure and Reactivity
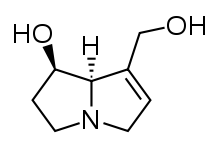
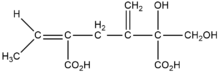
Riddelliine is a naturally occurring pyrrolizidine alkaloid, a class of compounds occurring in rangeland plants of the genera Crotalaria, Amsinckia, and Senecio.[7] It consists of a macrocyclic diester of retronecine (an unsaturated alcohol) (Figure 1) and riddelliic acid (an oxygenated, branched, dicarboxylic acid)(Figure 1).[7] Riddelliine is a colorless to off-white crystalline solid at room temperature and has a melting point of 197° to 198 °C.[5] It is soluble in chloroform, acetone, and ethanol, and is sparingly soluble in water. As a solid, it is stable at room temperature in diffuse light for 12 months or longer. Alcoholic and aqueous solutions of riddelliine are stable at room temperature when protected from light.[7] It emits toxic fumes of nitrogenoxide when heated to decomposition.[5]
Synonyms: 13,19-didehydro-12,18-dihydroxy senecionan-11,16-dione; trans-15-ethylidine-12b-hydroxy-12a-hydroxymethyl-13- methylenesenec-1-enine; 3-ethylidine-3,4,5,6,9,11,13,14,14a,14b-decahydro-6-hydroxy-6- (hydroxymethyl)-5-methylene(1,6)di-oxacyclododecino (2,3,4-gh)-pyrrolizidine-2,7-dione.[7]
Synthesis
Riddelliine is naturally produced by the plants of the genus Senecio which can be found in the rangelands in the western United States. The highest concentration of the alkaloids can be found in the seeds and de flowering tops of the plant.[7] The extraction starts by filtering the ground plant with ethanol during forty-eight hours. After the ethanol is removed, ether and chloroform is added. After this mixture is distilled, there is crude, crystalke riddelliine left. Further purification can be done with absolute ethanol.[6]
No methods for chemically synthesizing Riddelliine have been established this moment in time.
Available Forms
The plant’s containing Riddelliine are growing in a big variety in nature and are in some extend a danger to cattle.[8] Plants that contain Riddelliine are available for analytical use.[9]
Mode of action
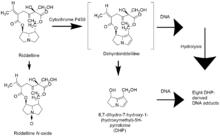
There are two possible pathways that lead to DHP- derived DNA adducts, which induce liver tumor formation. The first is the covalent binding of dehydroriddelliine to cellular DNA followed by hydrolysis, either enzymatically or chemically, to the eight DHP-derived DNA adducts (Figure "Oxidative Metabolism of Riddelliine in Rat liver").[10]
The second pathway is that dehydroriddelliine is hydrolyzed and catalyzed by esterases and/or other hepatic enzymes, to form DHP. DHP subsequently binds covalently to DNA so that DHP-derived mono and dinucleotide DNA adducts are formed. Till now there is no evidence on which of the two pathways predominates the DNA adduct formation.
Metabolism
Riddelliine is biotransformed in the liver.[10] The formation of DHP and Riddelliine N-oxide resulting from the metabolism of Riddelliine is catalyzed by the cytochrome P450 enzyme.[10] The metabolism of Riddelliine first provides 8-hydroxyriddelliine and/or 3-hydroxyriddelliine as the primary metabolites. Through enzymatic dehydration of the primary metabolites the reactive metabolites, R- and S- dehydroriddelliine (DHP)(6,7-dihydro-7hydroxy-1-(hydroxymethyl)-5H-pyrrolizine) are produced (Figure "Oxidative Metabolism of Riddelliine in Rat liver").[10] This enzymatic reactions involves the cytochrome P450 isozymes CYP3A and CYP2B6.[5] The dehydration pathway is considered to be cytotoxic whereas the oxidative metabolism of Riddelline, where it is hydrolyzed and converted into Riddelliine N-oxide, is detoxicating.
Indications
In rats gavaged with Riddelliine, a set of DNA adducts of dehydroretronecine (DHS) in liver DNA can serve as biomarkers for the tumorigenicity induced by Riddelline and related pyrrolizidine alkaloids.[11]
Efficacy
Plants containing Riddelliine, such as Senecio longilobus, S. jacobea or S. vulgaris, are used in medicinal herb preparations.[10]
Particularly in Jamaica, ‘bush tea’ is a treatment for children against a cold. ‘Gordolobo yerba’ is a herbal tea which is consumed in the south-west USA.[12]
Adverse effects
Diseases
In humans, acute hepatic veno-occlusive disease was observed after the consumption of a herbal preparation containing Riddelliine.[12]
Genotoxicity
In an in-vitro system, exposure to Riddelline caused sister chromatid exchange in human lymphocytes, DNA-protein cross linking in bovine kidney epithelial cells and gene mutations in bacteria.[12]
Carcinogenicity
There are no data on the carcinogenicity of Riddelliine to humans, but based on experimental animal studies, Riddelliine is classified as a Group 2B compound, which means that it is possibly carcinogenic to humans.[12]
Experimental animal studies
In rats, oral administration led to an increase in haemangiosarcomas in the liver, cellular carcinomas and/or adenomas in the liver and mononuclear cell leukemia.[12]
In mice, oral administration led to haemangiosracomas in the liver in males and to broncho-alveolar adenomas and carcinomas in females.[12]
In rodents in general, Riddelline disturbs the estrus cycle.[12]
In calves, a 100% morbidity rate after being fed a dose of 40.5 mg/kg bodyweight of Riddelline N-oxide was observed. After administration of doses of 15 – 20 mg/kg/day for 20 days, N-oxide caused seneciosis in cattle. Main clinical signs hereof are malaise, depression, erratic or unpredictable behavior, aimless walking and ataxia. Gross pathological finding are lesions in liver tissues and the central nervous system.[12]
Toxicity
Human exposure to pyrrolizidine alkaloids occurs through consumption of herbal dietary supplements, including comfrey, and through contaminated livestock products (e.g., milk).[13] Pyrrolizidine alkaloids (PAs) are probably the most common plant constituents that poison livestock, wildlife, and humans worldwide. Riddelliine is isolated from plants grown in the western United States and is a prototype of genotoxic PAs.[14] Riddelliine has been shown to have clear evidence of carcinogenic activity in male and female rats but there are no human studies done on the effects of Riddelliine on humans.[7] There have been cases of human poisoning due to pyrrolizidine alkaloids intake. These cases consists of the following incidents. Heliotrope poisoning is endemic in central Asia, where seeds of Heliotropium species enter the wheat crop. The typical clinical picture is that of ascites, hepatosplenomegaly, veno-occlusive disease of the liver, and abnormal liver function.[7] In South Africa, a disease known as "bread poisoning," found among poor Europeans, was traced to the inclusion of Senecio and Crotalaria seeds and flowers in whole grain processed for bread flour.[7] A veno-occlusive disease outbreak in Jamaica was traced to the widespread use of "bush tea," an herbal medicine used to treat children for colds.[7] In the Southwest of the United States, the popular herbal tea, gordolobo yerba, is a potential hazard for exposure to toxic PAs.[7]
Figure 1: Oxidative Metabolism of Riddelliine in Rat liver.
Effects on animals
Riddelliine is toxic to animals. The way animals are usually exposed to Riddelliine is via ingesting. It has been found in Crotalaria and Senecio species.[13] It has carcinogenic effect on rats and mice and it has been shown to induce liver hemangiosarcomas in rats and mice.[15] Furthermore, during an 5 and 30 day study, where the rats and mice where force fed Riddelliine, there was unscheduled DNA synthesis in the cultured hepatocytes in as well male as female rats and mice.[15]
It also increases the mutations in endothelial cells in the liver of rats.
Riddelliine has a characteristically nucleobases transversion of G:C to T:A. And it was highted from 9% in the control group to 17% in the treated rats.
In contrast, G:C→A:T transition, the major mutation in control rats where it made up 54% of all mutations, was reduced to 40% of mutations in riddelliine-treated rats.
These results suggest that the relatively high mutagenicity of riddelliine in rat liver endothelial cells may be partially responsible for the tumorigenic specificity of this agent.[14]
It is not only toxic to rodents but also to bacteria, more specific the Salmonella bacteria S. Typhimurium. When this bacteria is exposed to Riddelliine, it causes mutations in the genetic strains.[7]
Clinical signs in poisoned animals include neurological, gastrointestinal (diarrhea), and hematologic (high blood ammonia, hemolysis) effects. Ascites is often observed. Molyneux et al. (1991)[8] reported that calves fed Senecio riddellii, which contains only riddelliine and its N-oxide, for 20 days showed weight loss, signs of depression, reduced feed intake, ataxia of hind limbs, ascites, and edema before death.
Microscopic examination revealed hepatocellular necrosis and collapse of lobules, increased numbers of fibroblasts and collagen, portal edema, anisokaryosis of hepatocyte nuclei with some cytomegaly, and bile duct proliferation.[7]
See also
- Senecionine, a closely related pyrrolizidine alkaloid
References
- ↑ Adams, I. Roger; Hamlin, K. E., Jr.; Jelinek, C. F.; Phillips, R. F. (1942). "Structure of riddelliine, the alkaloid in Senecio riddellii ". Journal of the American Chemical Society. 64 (12): 2760–2763. doi:10.1021/ja01264a013.
- ↑ NTP Technical Report on Toxicity Studies of Riddelliine, National Toxicology Program, Toxicity Report Series, Number 27
- ↑ National Toxicology, Program (2003). "Toxicology and carcinogenesis studies of riddelliine (CAS No. 23246-96-0) in F344/N rats and B6C3F1 mice (gavage studies)". National Toxicology Program technical report series (508): 1–280. PMID 12844193.
- ↑ 12th Report on Carcinogens, National Toxicology Program
- 1 2 3 4 Manske, Richard H. F. (1939-01-01). "The Alkaloids of Senecio Species: Iii. Senecio Integerrimus, S. Longilobus, S. Spartioides and S. Ridellii". Canadian Journal of Research. 17b (1): 1–7. doi:10.1139/cjr39b-001. ISSN 1923-4287.
- 1 2 Adams, Roger; Hamlin, K. E.; Jelinek, C. F.; Phillips, R. F. (2002-05-01). "The Structure of Riddelliine, the Alkaloid in Senecio Riddellii. I". Journal of the American Chemical Society. 64 (12): 2760–2763. doi:10.1021/ja01264a013.
- 1 2 3 4 5 6 7 8 9 10 11 12 Chan, P.C. (1993). NTP Technical Report on toxicity studies of riddelliine, administered by gavage to F344/N rats and B6C3F mice. NIH.
- 1 2 Johnson, A. E.; et al. (1985). "Toxicity of Riddell's groundsel (Senecio riddellii) to cattle". ncbi. American Journal of Veterinary Research. Retrieved 2016-03-09.
- ↑ Sigma-Aldrich. "Comfrey (Symphytum officinale)". Sigma-Aldrich. Sigma-Aldrich. Retrieved 2016-03-09.
- 1 2 3 4 5 NTP (National Toxicology Program) (2014). Report on Carcinogens, 13th edition. Durham: U.S. Department of Health and Human Services Secretary.
- ↑ Yu-Ping Wang, P.P. (2005). Metabolic Activation of the Tumorigenic Pyrrolizidine Alkaloid, Retrorsine, Leading to DNA Adduct Formation In Vivo. Jefferson: National Center for Toxicological Research.
- 1 2 3 4 5 6 7 8 IARC Working Group On The Evaluation Of Carcinogenic Risks To Humans (2002). Some traditional Herbal Medicines, Some Mycotoxins, Naphtalene and Styrene. Lyon: World Health Organization: International Agency for Research on Cancer.
- 1 2 Williams, Lee; Chou, Ming W.; Yan, Jian; Young, John F.; Chan, Po C.; Doerge, Daniel R. (2002-07-15). "Toxicokinetics of Riddelliine, a Carcinogenic Pyrrolizidine Alkaloid, and Metabolites in Rats and Mice". Toxicology and Applied Pharmacology. 182 (2): 98–104. doi:10.1006/taap.2002.9441.
- 1 2 Mei, Nan; Guo, Lei; Liu, Ruqing; Fuscoe, James C; Chen, Tao (2007-11-01). "Gene expression changes induced by the tumorigenic pyrrolizidine alkaloid riddelliine in liver of Big Blue rats". BMC Bioinformatics. 8 (Suppl 7). doi:10.1186/1471-2105-8-s7-s4.
- 1 2 Chan, Po C. (2003). Toxicity and carcinogenicity of riddelliine in rats and mice. Toxicology Letters. pp. 295–311.