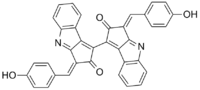
Scytonemin
 | |
| Names | |
|---|---|
| IUPAC name
(3E,3'E)-3,3'-bis(4-hydroxybenzylidene)-[1,1'-bi(cyclopenta[b]indole)]-2,2'(3H,3'H)-dione | |
| Other names
Scytonemin | |
| Identifiers | |
| 152075-98-4 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:90127 |
| ChEMBL | ChEMBL505177 |
| ChemSpider | 16736974 |
| |
| |
| Properties | |
| C36H20N2O4 | |
| Molar mass | 544.6 g/mol |
| Appearance | brown solid[1] |
| Solubility | 25mg/ml DMSO |
| UV-vis (λmax) | 325-425nm |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Scytonemin is a biological pigment synthesized by many strains of cyanobacteria, including Calothrix sp.,[2] Lyngbya aestuarii,[3] and others. It was originally discovered in 1849, although the structure remained unsolved until 1993.[4] Scytonemin is believed to act as a bacterial sunscreen with a broad absorption from 325-425 nm and a separate maxima at 250 nm,[4] and its biosynthesis triggered by exposure to UV light.[5]
Biosynthesis
The biosynthesis in Lyngbya aestuarii was recently explored by Balskus, Case, and Walsh. It proceeds by the conversion of L-tryptophan to 3-indole pyruvic acid, followed by coupling to p-hydroxyphenylpyruvic acid. Cyclization of the resultant β-ketoacid yields a tricyclic ketone. Oxidation and dimerization yields the completed natural product. Three scytonemin biosynthetic enzymes are necessary, denoted as ScyA-C.[3]

References
- ↑ "Scytonemin CAS (152075-98-4)". Santa Cruz Biotechnology, Inc. Retrieved 2011-05-30.
- ↑ Dillon, Jesse G.; Castenholz, Richard W. (2003). "The synthesis of the UV-screening pigment, scytonemin, and photosynthetic performance in isolates from closely related natural populations of cyanobacteria (Calothrix sp.)". Environmental Microbiology. 5 (6): 484–91. doi:10.1046/j.1462-2920.2003.00436.x. PMID 12755715.
- 1 2 3 Balskus, Emily P.; Case, Rebecca J.; Walsh, Christopher T. (2011). "The biosynthesis of cyanobacterial sunscreen scytonemin in intertidal microbial mat communities". FEMS Microbiology Ecology: 1–11. doi:10.1111/j.1574-6941.2011.01113.x.
- 1 2 Proteau, P. J.; Gerwick, W. H.; Garcia-Pichel, F.; Castenholz, R. (1993). "The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria". Experientia. 49 (9): 825–9. doi:10.1007/BF01923559. PMID 8405307.
- ↑ M. Bandaranayake, Wickramasinghe (1998). "Mycosporines: are they nature's sunscreens?". Natural Product Reports. 15 (2): 159–72. doi:10.1039/A815159Y. PMID 9586224.
External links
- Balskus, Emily P.; Case, Rebecca J.; Walsh, Christopher T. (2011). "The biosynthesis of cyanobacterial sunscreen scytonemin in intertidal microbial mat communities". FEMS Microbiology Ecology: no–no. doi:10.1111/j.1574-6941.2011.01113.x.