Silyl hydride
Silicon hydrides are chemical compounds which contain a silicon–hydrogen bond. The silicon-to-hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more electronegative than silicon (hence the naming convention of silyl hydrides), which results in the polarization of the Si-H bond to be the reverse of that for the C-H bond. Generally silyl hydrides are colourless with physical properties (solubility, volatility) comparable to hydrocarbons. They can be pyrophoric, reflecting the great driving force for replacing Si-H bonds with Si-O bonds.
The parent compound SiH4 is called silane. This colourless, flammable gas is widely used as a precursor to silicon films of interest in microelectronics. In the laboratory, most silicon hydrides contain organic substituents. One example is phenylsilane (PhSiH3).
Reactions and applications
Precursors to silicon coatings and films
The dominant application of silicon hydrides is in the production of silicon films and coatings. Silane decomposes as follows:
- SiH4 → Si + 2 H2
This reaction is conducted by chemical vapor deposition, exploits the weakness of the Si-H bond. The second most heavily produced silicon hydride is trichlorosilane, HSiCl3.[1]
Hydrosilylation
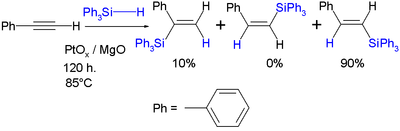
Silyl hydrides react with various unsaturated substrates such as alkenes, alkynes, imines, carbonyls and oximes to new organosilicon compounds in hydrosilylation. In the reaction of triphenylsilyl hydride with phenylacetylene the reaction product is a trans or cis or the geminal vinyl silane, for example:[2]
Laboratory reductants
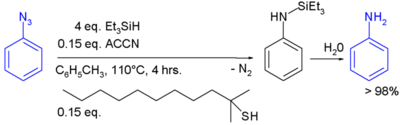
In the laboratory, silyl hydrides are used as reducing agent. For example, PMHS. In one study triethylsilane is used in the conversion of an phenyl azide to an aniline:[3]
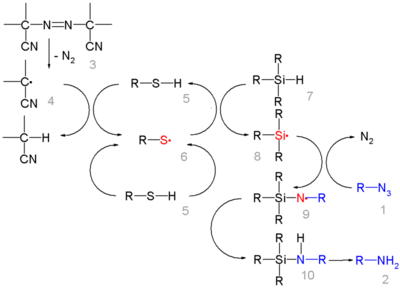
In this reaction ACCN is a radical initiator and an aliphatic thiol transfers radical character to the silylhydride. The triethylsilyl free radical then reacts with the azide with expulsion of nitrogen to a N-silylarylaminyl radical which grabs a proton from a thiol completing the catalytic cycle:
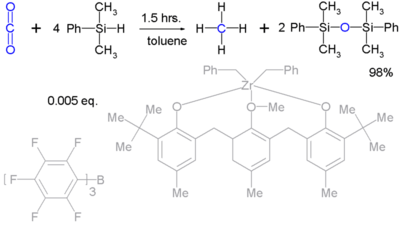
Silyl hydrides can reduct robust molecules such as carbon dioxide (to methane):[4] Unfortunately such reactions are stoichiometric and practical means to regenerate the silicon hydrides are not available.
In the related silylmetalation, a metal replaces the hydrogen atom.
References
- ↑ Simmler, W. (2005), "Silicon Compounds, Inorganic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a24_001
- ↑ Effect of the synthetic method of Pt/MgO in the hydrosilylation of phenylacetylene Eulalia Ramírez-Oliva, Alejandro Hernández, J. Merced Martínez-Rosales, Alfredo Aguilar-Elguezabal, Gabriel Herrera-Pérez, and Jorge Cervantesa Arkivoc 2006 (v) 126-136 Link
- ↑ Benati, Luisa; Bencivenni, Giorgio; Leardini, Rino; Minozzi, Matteo; Nanni, Daniele; Scialpi, Rosanna; Spagnolo, Piero; Zanardi, Giuseppe (2006). "Radical Reduction of Aromatic Azides to Amines with Triethylsilane". J. Org. Chem. 71 (15): 5822–5825. doi:10.1021/jo060824k.
- ↑ From Carbon Dioxide to Methane: Homogeneous Reduction of Carbon Dioxide with Hydrosilanes Catalyzed by Zirconium-Borane Complexes Tsukasa Matsuo and Hiroyuki Kawaguchi J. Am. Chem. Soc.; 2006; 128, pp 12362 - 12363; doi:10.1021/ja0647250