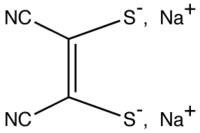
Sodium maleonitriledithiolate
 | |
| Names | |
|---|---|
| IUPAC name
sodium cis-1,2-dicyano-1,2-ethylenedithiolate | |
| Other names
sodium mnt sodium maleonitriledithiolate | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 5013443 |
| PubChem | 6523934 |
| |
| |
| Properties | |
| C4N2Na2S2 | |
| Molar mass | 186.17 g/mol |
| Appearance | yellow solid |
| Solubility in ethanol, DMF | Soluble |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Sodium maleonitriledithiolate is the chemical compound described by the formula Na2S2C2(CN)2. The name refers to the cis compound, formally derived from maleonitrile. This is the most common salt of the dianion, maleonitriledithiolate, often called mnt, which is a "dithiolene", i.e. a chelating alkene-1,2-dithiolate that serves as a non-innocent ligand for transition metals and is commonly used in coordination chemistry. Several complexes are known, such as [Ni(mnt)2]2−.[1]:143-146
The salt is synthesized by treating carbon disulfide with sodium cyanide to give the cyanodithioformate salt, which eliminates elemental sulfur in aqueous solution:[2]
- 2 NaCN + 2 CS2 → Na2S2C2(CN)2 + 1/4 S8
The compound was first described by Bähr and Schleitzer 1958.[3]
References
- ↑ Day, P. and Coronado, E. (2004) Molecular Materials Combining Magnetic and Conducting Properties, in Magnetism: Molecules to Materials V (eds J. S. Miller and M. Drillon), Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, FRG. doi: 10.1002/3527604383.ch4
- ↑ R. H. Holm, A. Davison "Metal Complexes Derived from cis-1,2-Dicyano-1,2-Ethylenedithiolate and Bis(trifluoromethyl)-1,2-Dithiete" Inorganic Syntheses 1967, volume X, pp.8-26.
- ↑ G. Bähr and G. Schleitzer (1957). "Beiträge zur Chemie des Schwefelkohlenstoffs und Selenkohlenstoffs, II. Die Kondensierende Spontan-Entschwefelung von Salzen und Estern der Cyan-Dithioameisensäure. Freie Cyan-Dithioameisensäure". Chemische Berichte. 90 (3): 438–443. doi:10.1002/cber.19570900322.
This article is issued from Wikipedia - version of the 2/24/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.