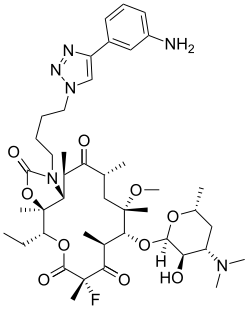
Solithromycin
 | |
| Clinical data | |
|---|---|
| Routes of administration | oral, intravenous |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | CEM-101; OP-1068 |
| CAS Number |
760981-83-7 |
| ChemSpider |
25056854 |
| UNII |
9U1ETH79CK |
| ChEMBL |
CHEMBL1240704 |
| Chemical and physical data | |
| Formula | C43H65FN6O10 |
| Molar mass | 845.01 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Solithromycin (formerly known as CEM-101 and OP-1068) is a ketolide antibiotic undergoing clinical development for the treatment of community-acquired pneumonia (CAP)[1] and other infections.[2]
Solithromycin exhibits excellent in vitro activity against a broad spectrum of Gram-positive respiratory tract pathogens,[3][4] including macrolide-resistant strains.[5] Solithromycin has activity against most common respiratory Gram-(+) and fastidious Gram-(-) pathogens,[6][7] and is being evaluated for its utility in treating gonorrhea.
- May 2011: Solithromycin is in a Phase 2 clinical trial for serious community-acquired bacterial pneumonia (CABP) and in a Phase 1 clinical trial with an intravenous formulation.[8]
- September 2011 : Solithromycin demonstrated comparable efficacy to levofloxacin with reduced adverse events in Phase 2 trial in people with community-acquired pneumonia[9]
- January 2015: In a Phase 3 clinical trial for community-acquired bacterial pneumonia (CABP), Solithromycin administered orally demonstrated statistical non-inferiority to the fluoroquinolone, Moxifloxacin.[10]
- July 2015: Patient enrollment for the second Phase 3 clinical trial (Solitaire IV) for community-acquired bacterial pneumonia (CABP) was completed with results expected in Q4 2015.[11]
- Oct 2015: IV to oral solithromycin demonstrated statistical non-inferiority to IV to oral moxifloxacin in adults with CABP.[12]
- July 2016: Cempra Announces FDA Acceptance of IV and oral formulations of Solithera (solithromycin) New Drug Applications for in the Treatment of Community-Acquired Bacterial Pneumonia.[13]
Structure
X-ray crystallography studies have shown solithromycin, the first fluoroketolide in clinical development, has a third region of interactions with the bacterial ribosome,[14] as compared with two binding sites for other ketolides.
The only currently marketed ketolide, telithromycin, suffers from rare, but serious side effects. Recent studies[15] have shown this to be likely due to the presence of the aniline-triazole group of the telithromycin side chain acting as an antagonist towards various nicotinic acetylcholine receptors.
References
- ↑ Reinert RR (June 2004). "Clinical efficacy of ketolides in the treatment of respiratory tract infections". The Journal of Antimicrobial Chemotherapy. 53 (6): 918–27. doi:10.1093/jac/dkh169. PMID 15117934.
- ↑ http://www.cempra.com/research/antibacterials/
- ↑ Woolsey LN; Castaneira M; Jones RN. (May 2010). "CEM-101 activity against Gram-positive organisms". Antimicrobial Agents and Chemotherapy. 54 (5): 2182–2187. doi:10.1128/AAC.01662-09. PMID 20176910.
- ↑ Farrell DJ; Sader HS; Castanheira M; Biedenbach DJ; Rhomberg PR; Jones RN. (June 2010). "Antimicrobial characterization of CEM-101 activity against respiratory tract pathogens including multidrug-resistant pneumococcal serogroup 19A isolates". International Journal of Antimicrobial Agents. 35 (6): 537–543. doi:10.1016/j.ijantimicag.2010.01.026. PMID 20211548.
- ↑ McGhee P; Clark C; Kosowska-Shick K; Nagai K; Dewasse B; Beachel L; Appelbaum PC. (January 2010). "In Vitro Activity of Solithromycin against Streptococcus pneumoniae and Streptococcus pyogenes with Defined Macrolide Resistance Mechanisms". Antimicrobial Agents and Chemotherapy. 54 (1): 230–238. doi:10.1128/AAC.01123-09. PMC 2798494
 . PMID 19884376.
. PMID 19884376. - ↑ Putnam, Shannon D.; Castanheira, Mariana; Moet, Gary J.; Farrell, David J.; Jones, Ronald N. (2010). "CEM-101, a novel fluoroketolide: antimicrobial activity against a diverse collection of Gram-positive and Gram-negative bacteria". Diagnostic Microbiology and Infectious Disease. 66 (4): 393–401. doi:10.1016/j.diagmicrobio.2009.10.013. PMID 20022192.
- ↑ Putnam, Shannon D.; Sader, Helio S.; Farrell, David J.; Biedenbach, Douglas J.; Castanheira, Mariana (2011). "Antimicrobial characterisation of solithromycin (CEM-101), a novel fluoroketolide: activity against staphylococci and enterococci". International Journal of Antimicrobial Agents. 37 (1): 39–45. doi:10.1016/j.ijantimicag.2010.08.021. PMID 21075602.
- ↑ "Intravenous (IV) Administration of Cempra Pharmaceutical's Solithromycin (CEM-101) Demonstrates Excellent Systemic Tolerability in a Phase 1 Clinical Trial". 7 May 2011.
- ↑ "Cempra antibiotic compound as effective, safer than levofloxacin". 15 Sep 2011.
- ↑ http://investor.cempra.com/releasedetail.cfm?ReleaseID=889300. 4 Jan 2015
- ↑ http://investor.cempra.com/releasedetail.cfm?ReleaseID=920866. 7 July 2015
- ↑ http://investor.cempra.com/releasedetail.cfm?ReleaseID=936994
- ↑ http://investor.cempra.com/releasedetail.cfm?ReleaseID=978096
- ↑ Llano-Sotelo B, Dunkle J, Klepacki D, Zhang W, Fernandes P, Cate JH, Mankin AS (2010). "Binding and Action of CEM-101, a New Fluoroketolide Antibiotic That Inhibits Protein Synthesis". Antimicrobial Agents and Chemotherapy. 54 (12): 4961–4970. doi:10.1128/AAC.00860-10. PMC 2981243
 . PMID 20855725.
. PMID 20855725. - ↑ Bertrand D, Bertrand S, Neveu E, Fernandes P (2010). "Molecular characterization of off-target activities of telithromycin: a potential role for nicotinic acetylcholine receptors". Antimicrobial Agents and Chemotherapy. 54 (12): 599–5402. doi:10.1128/AAC.00840-10. PMC 2981250
 . PMID 20855733.
. PMID 20855733.