Tentoxin
 | |
| Names | |
|---|---|
| IUPAC names
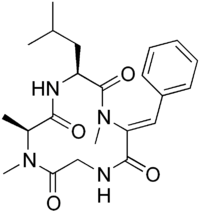
Cyclo(N-methyl-L-alanyl-L-leucyl-alpha,beta- didehydro-N-methylphenylalanylglycyl) | |
| Identifiers | |
| 28540-82-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4444584 |
| PubChem | 5281143 |
| |
| |
| Properties | |
| C22H30N4O4 | |
| Molar mass | 414.498 g/mol |
| Melting point | 172 to 175 °C (342 to 347 °F; 445 to 448 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tentoxin is a natural cyclic tetrapeptide produced by phytopathogenic fungus Alternaria alternata. It selectively induces chlorosis in several germinating seedling plants. Therefore, tentoxin may be used as a potential natural herbicide.
Tentoxin was first isolated from Alternaria alternata (syn. tenuis) and characterized by George Templeton et al. in 1967.[1]
Tentoxin has also been used in recent research to eliminate the polyphenol oxidase (PPO) activity from seedlings of higher plants.[2]
References
- ↑ Templeton, G. E., C. 1. Grable, N. D. Fulton, W. L. Meyer. 1967. Tentoxin from Alternaria tenuis: its isolation and characterization. Proceedings of the Mycotoxin Research Seminar, Washington, D. C., June 8–9, 1967. United States Department of Agriculture. pp. 27-29
- ↑ Duke, S.O. & Vaughn, K.C. 1982. Lack of involvement of polyphenol oxidase in ortho-hydroxylation of phenolic compounds in mung bean seedlings. Physiol. Plant. 54: 381-385.
External MSDS
- Tentoxin SDS from Fermentek
This article is issued from Wikipedia - version of the 10/30/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.