Triethylenetetramine
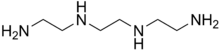 | |
 | |
 | |
| Names | |
|---|---|
| Other names
N,N'-Bis(2-aminoethyl)ethane-1,2-diamine; TETA; Trien; Trientine (INN); Syprine (brand name) | |
| Identifiers | |
| 112-24-3 | |
| 3D model (Jmol) | Interactive image |
| 605448 | |
| ChEBI | CHEBI:39501 |
| ChEMBL | ChEMBL609 |
| ChemSpider | 21106175 |
| ECHA InfoCard | 100.003.591 |
| EC Number | 203-950-6 |
| 27008 | |
| KEGG | C07166 |
| MeSH | Trientine |
| PubChem | 5565 |
| RTECS number | YE6650000 |
| UNII | SJ76Y07H5F |
| UN number | 2259 |
| |
| |
| Properties | |
| C6H18N4 | |
| Molar mass | 146.24 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Fishy, ammoniacal |
| Density | 982 mg mL−1 |
| Melting point | −34.6 °C; −30.4 °F; 238.5 K |
| Boiling point | 266.6 °C; 511.8 °F; 539.7 K |
| Miscible | |
| log P | 1.985 |
| Vapor pressure | <1 Pa (at 20 °C) |
| Refractive index (nD) |
1.496 |
| Thermochemistry | |
| 376 J K−1 mol−1 (at 60 °C) | |
| Hazards | |
| GHS pictograms |   |
| GHS signal word | DANGER |
| H312, H314, H317, H412 | |
| P273, P280, P305+351+338, P310 | |
| EU classification (DSD) |
|
| R-phrases | R21, R34, R43, R52/53 |
| S-phrases | (S1/2), S26, S36/37/39, S45 |
| Flash point | 129 °C (264 °F; 402 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
|
| Related compounds | |
| Related amines |
|
| Related compounds |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Triethylenetetramine, abbreviated TETA and trien and also called trientine (INN), is an organic compound with the formula [CH2NHCH2CH2NH2]2. This oily liquid is colorless but, like many amines, assumes a yellowish color due to impurities resulting from air-oxidation. It is soluble in polar solvents. The branched isomer tris(2-aminoethyl)amine and piperazine derivatives may also be present in commercial samples of TETA.[1]
Production
TETA is prepared by heating ethylenediamine or ethanolamine/ammonia mixtures over an oxide catalyst. This process gives a variety of amines, which are separated by distillation and sublimation.[2]
Uses
The reactivity and uses of TETA are similar to those for the related polyamines ethylenediamine and diethylenetriamine. It was primarily used as a crosslinker ("hardener") in epoxy curing.[2]
The hydrochloride salt of TETA, referred to as trientine hydrochloride, is a chelating agent that is used to bind and remove copper in the body to treat Wilson's disease, particularly in those who are intolerant to penicillamine. Some recommend trientine as first-line treatment, but experience with penicillamine is more extensive.[3]
Coordination chemistry
TETA is a tetradentate ligand in coordination chemistry, where it is referred to as trien.[4] Octahedral complexes of the type M(trien)Cl3 can adopt several diastereomeric structures, most of which are chiral.[5]
References
- ↑ "Ethyleneamines" (PDF). Huntsman. 2007.
- 1 2 Eller, K.; Henkes, E.; Rossbacher, R.; Höke, H. (2005). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001.
- ↑ Roberts, E. A.; Schilsky, M. L. (2003). "A practice guideline on Wilson disease" (pdf). Hepatology. 37 (6): 1475–1492. doi:10.1053/jhep.2003.50252. PMID 12774027.
- ↑ von Zelewsky, A. (1995). Stereochemistry of Coordination Compounds. Chichester: John Wiley. ISBN 047195599X.
- ↑ Utsuno, S.; Sakai, Y.; Yoshikawa, Y.; Yamatera, H. (1985). "Three Isomers of the Trans-Diammine-[N,N′-bis(2-Aminoethyl)-1,2-Ethanediamine]-Cobalt(III) Complex Cation". Inorganic Syntheses. 23: 79–82. doi:10.1002/9780470132548.ch16.
